Translate this page into:
Immunological detection assays for recombinant Shiga toxin & Shigella dysenteriae
For correspondence: Dr Pallavi Gupta, Biotechnology Division, Defence Research & Development Establishment, Gwalior 474 002, Madhya Pradesh, India e-mail: pallavi@drde.drdo.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Shiga toxin (Stx) is produced by Shigella dysenteriae, a Gram-negative, facultative anaerobic bacillus that causes shigellosis, haemolytic uraemic syndrome (HUS) and Reiter's syndrome. The detection methods for shiga toxin needs to be rapid, accurate, reliable and must be extensively evaluated under field conditions. The aim of this study was to develop rapid, sensitive and specific detection method for Stx.
Methods:
Mice and rabbits were immunized with purified recombinant Shiga toxin B (rStxB). Using these antibodies dot ELISA, sandwich ELISA and flow through assay were developed.
Results:
The high-titre antibodies specifically reacted with purified rStxB. Dot-ELISA, sandwich ELISA and flow-through assay were developed and standardized that could detect StxB with limit of detection (LOD) of 9.75, 9.7 ng/ml and 0.46 μg/cassette, respectively.
Interpretation & conclusions:
The rStxB was used to produce antibodies to avoid handling of pathogen. The Flow through assay ‘developed was specific, rapid and field amenable.
Keywords
Anti-Shiga toxin B serum
dot-enzyme-linked immunosorbent assay
flow-through assay
immunological assays
Shiga toxin
Shigella dysenteriae
Shigella dysenteriae type 1 remains the most virulent species of the genus Shigella and is involved in major epidemics1. It is the only member of the Shigella species that produces Shiga toxin (Stx) which has cytotoxic and neurotoxic properties. A key functional role of the StxB subunit is to mediate binding of Stx to surface receptors of susceptible cells2. Conventional microbiological techniques used for the detection, i.e. isolation and biochemical identification of the bacteria are time-consuming and laborious. Some methods may give reliable results in one single step, but usually, additional steps are necessary for confirmation3. The sensitivity and specificity of these methods depend strongly on the number of confirmatory tests performed that may take several days to complete. Shigella are usually isolated from faecal specimens by in vitro cultivation followed by identification by biochemical tests and agglutination assays45. This process usually requires 48-72 h. The bacteria invade and multiply in colonic epithelial cells, leading to colitis and bloody stools. S. dysenteriae serotype 1 produces Stx which shows several biological activities such as lethality to rabbits and mice. The toxin is cytotoxic to various cell lines including Vero cells and has exhibited enterotoxicity when injected into rabbit ileal loops67. Bioassay using Vero cells is available for the detection of Stx8. However, it requires culture facilities and is time-consuming, labour intensive and expensive. Colony blot assay has also been suggested9. In our previous study10, recombinant StxB subunit (7.7 kDa) was expressed and purified in native conditions. This protein was found to produce high-titre protective antibodies10. The present study was aimed to develop sandwich ELISA, dot-ELISA as well as flow-through assay for the detection of StxB.
Material & Methods
Immunization of mice and rabbits by recombinant Shiga toxin B (rStxB)
In our previous study10, the stxB gene (289 bp from 967 to 1255, accession number EF685161) coding for B chain of Stx was amplified. After cloning, StxB was expressed and purified in the native conditions11. BALB/c mice and New Zealand female white rabbits were maintained in animal care facility of Defence Research & Development Establishment (DRDE), Gwalior, India, at temperature 25°C±2°C and humidity 30-70 per cent with 12:12-h light:dark period. Animals were provided food obtained from Amrut Ltd., Chandigarh. The study was approved by the Institutional Animal Ethics Committee of DRDE. Route of immunization for mice was intraperitoneal and that for rabbits was intramuscular. Immunization was carried out on 0, 7, 14 and 21 days in BALB/c mice. One set (12 mice) was immunized with cross-linked recombinant Shiga toxin B (rStxB) and another set of four mice was immunized with rStxB without glutaraldehyde. The antigen was emulsified in Freund's complete adjuvant for the first dose, and on days 7, 14 and 21, Freund's incomplete adjuvant- based emulsion was used. The antigen was injected (10, 10, 20 and 40 μg/mice), respectively, as on the days mentioned above. The rabbits (n=3) were also immunized with the cross-linked rStxB. The rabbits were primed with the dose of 50 μg followed by the booster doses of 100 μg.
Collection of antiserum: Pre- immunized mice and rabbits were used as negative control. Initially, the test bleed was collected on the 10th day and final bleed on the 25th day of immunization. Mice were bled through retro- orbital route by inserting a microcapillary into the orbital plexus at an angle of 45°. The blood was collected through the capillary and pooled from each of the mice. From rabbits the blood was collected from ear vein for the test and by cardiac puncture for the final bleed. The collected blood was incubated at 37°C for 30 min and at 4°C for one hour. After dislodging and centrifugation at 6000×g for 10 min, antiserum was collected and stored at −80°C till further use.
Specificity of mouse and rabbit antiserum: Specificity of rabbit and mouse antiserum was checked against different toxins for specific detection of rStxB. Various toxins, i.e. rStxB, Staphylococcus enterotoxin B (SEB), Escherichia coli and Vibrio cholerae with the concentration of 500 ng/well in coating buffer (50 mM sodium carbonate-bicarbonate buffer, pH 9.6) (Sigma-Aldrich, USA) were coated on 96-well MaxiSorp microtitre plate (Nunc-Nalgene, USA). A dilution of 1:1000 of rabbit and mouse antiserum was prepared in one per cent bovine serum albumin (BSA); 100 μl of each was added in respective wells and incubated for one hour at 37°C, and absorbance was read at 450 nm using the ELISA plate reader (BioTek Instruments, USA).
Rabbit and mouse antiserum titre: The final bleed antiserum samples of mice and rabbits were subjected to the Western blot to check the titre. Two-fold serial dilutions of antiserum were prepared starting with 1:1000 up to 1:512,000 in phosphate-buffered saline (PBS). Standard Western blot protocol was performed as described elsewhere11. ELISA was performed in which antigen solution (500 ng) was prepared in coating buffer. The dilutions of mouse and rabbit antiserum ranging from 1:1000 to 1:2,048,000 were prepared in one per cent BSA in PBS. Pre-immunized serum was used as negative control. Subsequently, 100 μl of HRP (horse raddish peroxidase) conjugated anti-mouse and anti- rabbit immunoglobulin (1:2000) (Sigma-Aldrich, USA) were added to the respective wells and incubated for one hour at 37°C. Finally, the substrate 3,3’,5,5’-tetramethylbenzidine (TMB) (100 μl/well) was added, and the reaction was stopped with 50 μl (2N H2 SO4). Absorbance was read at 450 nm using ELISA plate reader. The cut-off value was calculated by the formula described by Snyder et al9, cut-off value = (average OD+standard deviation of blank ×3).
Sandwich ELISA for detection of Shiga toxin (Stx): Limit of detection (LOD) of rStxB was checked by sandwich ELISA using rabbit antiserum as capture antibody and mouse antiserum as revealing. Rabbit antiserum (1:1600) was coated (100 μl/well) on 96-well MaxiSorp microtitre plate in coating buffer (pH 9.6). The plate was incubated overnight at 4°C, after decanting blocking was done with three per cent BSA (2 h at 37°C). Washing was done thrice with PBS and tween-20 (PBST) and once with PBS. Two- fold serial dilutions from 500 ng/well of rStxB were prepared in one per cent BSA in PBS. One hundred microlitres/well was added and was incubated for one hour at 37°C. For the detection of Stx in S. dysenteriae type 1 (SD-1) and STEC (Shiga-like toxin (SLT)-producing E. coli), StxB was replaced with culture lysates. The wells containing one per cent BSA and pre-immune mouse serum served as the negative controls. BoNT/B, BoNT/E and ETX were used for specificity determination. Mouse anti-StxB antiserum (1:1600) was added and plate was incubated as above. After washing, 100 μl anti- mouse HRP immunoglobulin (1:5000) was added and incubated for one hour at 37°C. The substrate, TMB (100 μl/well), was added, the reaction was stopped and absorbance was read at described above. Food samples such as milk, paneer and apple juice were spiked with purified rStxB (1 μg/ml) and detected by sandwich ELISA as described above.
Dot-ELISA for detection of Shiga toxin B (StxB): Dot-ELISA was performed to determine LOD of StxB using nitrocellulose membrane (Micro Devices, India). Five microlitres of rabbit antiserum (1:250 in coating buffer) was placed on membrane of all eight strips of comb and left for drying at room temperature for 10 min. The membrane was incubated for one hour at 37°C in moist chamber. Skimmed milk powder (200 μl 5% in PBS) was used to block active sites. The membrane was washed thrice with PBST and once with PBS (5 min each). Two-fold serial dilutions of rStxB starting with 125 to 1.95 ng/well were prepared and 200 μl was added in the plate and the membrane was dipped. The last well containing one per cent BSA served as negative control. The membrane was washed as above and kept in plate containing mouse anti-StxB serum (1:500) for one hour at 37°C. After washing, membrane was incubated in anti-mouse HRP conjugate (1:2000). Substrate 3,3’-diaminobenzidine (DAB) + H2O2 was added after the washing and kept until the appearance of brown dots.
Flow-through assay for detection of Shiga toxin (Stx): Nitrocellulose membrane and absorbent pads were placed and fitted into small-sized cassettes (Micro Devices, India) used for performing flow-through assay in sandwich format. Mouse anti-StxB serum was mixed (1:1 v/v) in coating buffer and 2 μl was added on membrane. Pre-immunized rabbit serum (1:100) was added on the top of the membrane which served as positive control and incubated at 37°C for one hour. Non-specific sites were blocked by incubating at 37°C for one hour in three per cent BSA. The membrane was washed by soaking it in PBST followed by PBS. Two-fold serially diluted rStxB was added in different cassettes with concentrations of 15 to 0.46 μg/cassette and kept for two minutes. BSA (1%) was used as negative control. Subsequently, the membrane was washed by soaking in PBST and PBS. Purified rabbit immunoglobulin G (IgG) (500 ng/cassette) was added and kept for one minute, and after washing, rabbit HRP conjugate (1:5000) was added, kept for one minute and detected by adding DAB and H2O2.
Flow-through assay for detection of Shigella dysenteriae type 1: Cassettes were prepared as described above and mouse anti-StxB serum was mixed (500 ng) in coating buffer, and 2 μl was added on the membrane and incubated at 37°C for one hour. Pre-immunized rabbit serum (1:100) served as positive control. Non-specific sites were blocked by incubating with three per cent BSA. After washing SD-1 lysates of National Institute of Cholera and Enteric Diseases (NICED), Kolkata, and Christian Medical College (CMC), Vellore, cultures (1:10 in 1% BSA) were added in the respective cassettes and kept for two minutes. E. coli SG13009 lysate was used as negative control. After washing, purified rabbit IgG (500 ng/cassette) was added and kept for one minute, after washing, rabbit HRP conjugate (1:5000) was added, kept for one minute and substrate used for detection.
Results
Specificity and titre of anti-Shiga toxin B (StxB) serum: The rStxB was purified using affinity and gel filtration chromatography. After cross-linking rStxB was found to produce high-titre protective antibodies in mice as reported in our previous study11. The rabbit and mouse antisera were analyzed for the cross-reactivity with other toxins, i.e. SEB, E. coli and V. cholerae. Results showed that the anti-StxB rabbit serum was highly specific for StxB, and no cross-reactivity was observed (Fig. 1). Mouse antiserum was also found to be specific (data not shown).

- Specificity of rabbit anti-recombinant Shiga toxin B serum by ELISA. Graph represents interaction of anti-recombinant Shiga toxin B rabbit serum against recombinant Shiga toxin B (rStx B), Staphylococcus enterotoxin B (SEB), Escherichia coli (E. coli) and Vibrio cholerae (V. cholerae).
Rabbit and mouse antiserum titre against rStxB by Western blot and ELISA: Cross-linked rStxB was used for immunization of rabbits and mice, and titres of antisera were estimated by Western blot and ELISA. The titre estimated by Western blot for the rabbit antiserum was 1:256,000 (Fig. 2A), and in ELISA, the titre was 1:1,024,000 with the cut-off value of 0.15 (Fig. 2B). Similarly, the titre estimated by Western blot for mouse antisera was 1:256,000 (Fig. 2C), and in ELISA, it was 1:512,000 where the cut-off value was 0.12 (Fig. 2D).
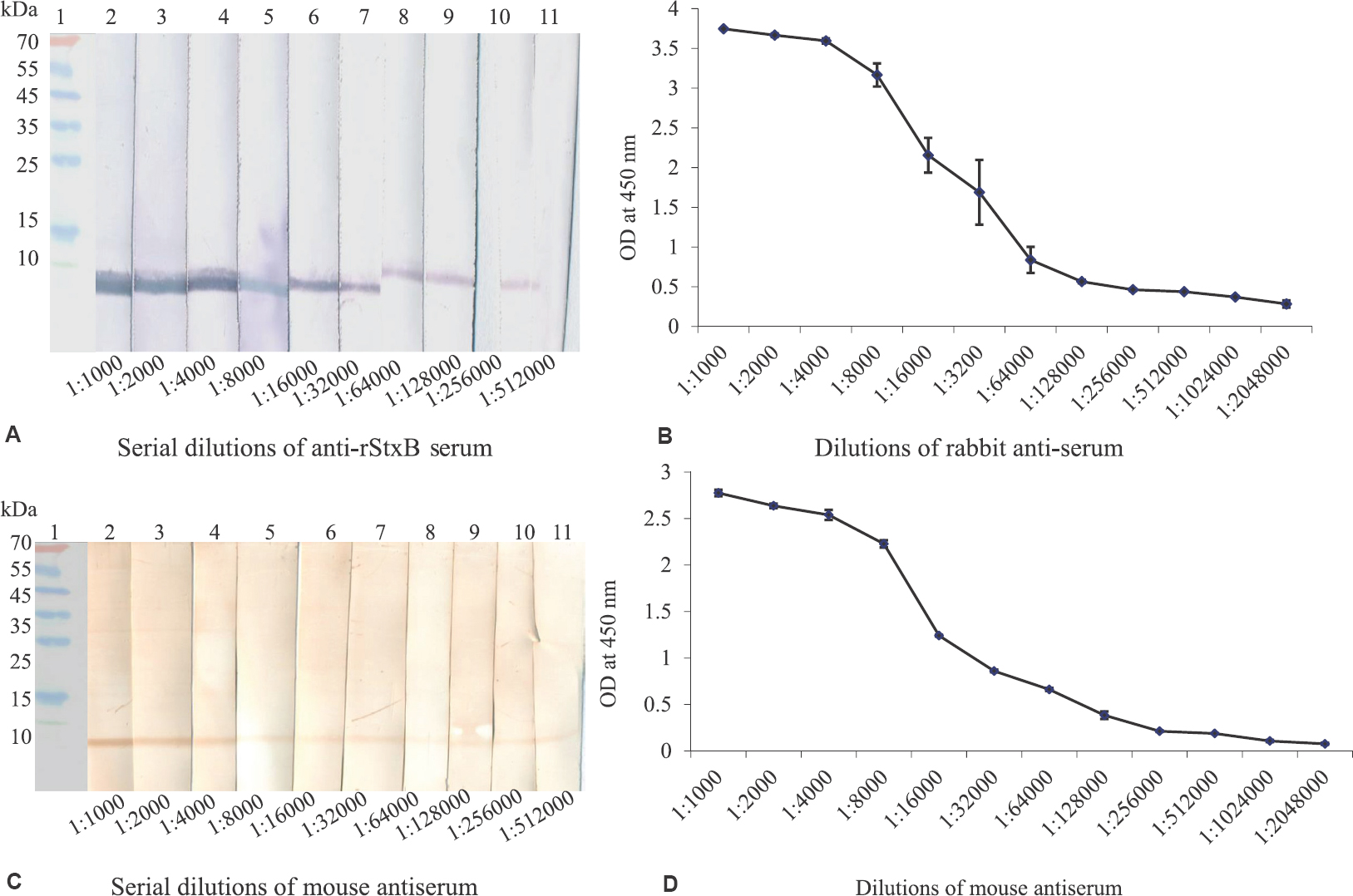
- (A) Western blot showing titre of rabbit anti-recombinant Shiga toxin B serum. Lane 1: pre-stained molecular weight marker (kDa); lanes 2-11: two-fold serial dilutions (1:1000 to 1:512,000) of anti-recombinant Shiga toxin B rabbit serum. (B) ELISA showing titre of rabbit-anti-recombinant Shiga toxin B serum. Graph showing two-fold serial dilutions of anti-recombinant Shiga toxin B rabbit serum. Blue line represents interaction of recombinant Shiga toxin B with antiserum; cut-off value is 0.15. (C) Western blot showing titre of mouse anti-recombinant Shiga toxin B serum. Lane 1: pre-stained molecular weight marker (kDa); lanes 2-11: two-fold serial dilutions (1:1000 to 1:512000) of anti-recombinant Shiga toxin B mouse serum. (D) ELISA showing titre of mouse anti-recombinant Shiga toxin B serum. Graph represents two-fold serial dilutions of anti-recombinant Shiga toxin B mouse serum; blue line represents interaction of recombinant Shiga toxin B with antiserum. Cut-off value is 0.12.
Sensitivity test of rStxB by sandwich ELISA: The LOD of rStxB achieved was 9.7 ng/ml. The data suggested that antibodies produced against rStxB were sensitive and suitable for use as diagnostic reagents (Fig. 3A), where solid rectangular represents negative control and hollow rectangular is rStxB. The detection of SD-1and STEC SLT-producing E. coli b culture lysates was also done by sandwich ELISA (Fig. 3B). The cut-off value was 0.3. Anti-StxB sera did not react with the negative controls. The sandwich ELISA was found to detect rStxB in various spiked food samples (unpublished data).
- (A) Sandwich ELISA showing limit of detection of recombinant Shiga toxin B (rStxB) using rabbit and mouse antiserum used for capture and revealing, respectively. Graph represents concentration (ng/ml) of rStxB (hollow rectangular) and negative control (solid rectangular). Cut-off value was 0.3. (B) Sandwich ELISA for detection of Shiga toxin from culture lysates. Graph represents reactivity of recombinant Shiga toxin B antiserum with rStxB, Shiga like toxin producing Escherichia coli (E. coli )(STEC), Shigella dysenteriae type 1, E. coli (SG12009) and Phosphate buffered saline (PBS).
Sensitivity test of rStxB by dot-ELISA: The sensitivity test was performed to check the detection limit of by dot-ELISA. LOD of StxB was found to be 9.75 ng/ml (Fig. 4).
- Dot-ELISA for limit of detection of recombinant Shiga toxin B. Each well showing two-fold serially diluted concentration of recombinant Shiga toxin B from 125 to 1.95 ng/well; last well is negative control.
Sensitivity test of rStxB by flow-through assay: Rapid detection of Stx was carried out by flow-through assay within five minutes. Substrate addition led to the appearance of brown dots in rStxB and produced Stx. LOD of rStxB was found to be 0.46 μg as shown in Fig. 5 (cassette no. 6). Stx present in culture lysates of SD-1 and STEC was also detected by flow-through assay using anti-StxB serum within five minutes (Fig. 6). Negative controls remained colourless in both the cases.
- Detection of recombinant Shiga toxin B by rapid flow-through assay. Cassette nos. 1-6 contain two-fold serially diluted concentration of recombinant Shiga toxin B (μg/cassette) from 15 to 0.46 μg/cassette; the last cassette represents negative control.
- Detection of Shigella dysenteriae culture lysates by rapid flow-through assay. Upper dot represents positive controls. Capture antibody anti-recombinant Shiga toxin B-purified mouse immunoglobulin G (500 ng/cassette); revealing antibody anti-recombinant Shiga toxin B-purified rabbit immunoglobulin G 500 ng/cassette) Cassette no.1. Shigella dysenteriae culture lysate from Christian Medical College, Vellore; 2. Shigella dysenteriae culture lysate from of National Institute of Cholera and Enteric Diseases, Kolkata; 3. Escherichia coli SG13009 (negative control).
Discussion
Shiga toxins are bipartite molecules of two polypeptide chains, an A subunit (32 k Da) which non-covalently associates with 7.7 kDa pentameric B subunit12. The aim of this study was to develop field-based rapid immunological assays. StxB subunit was chosen, as it is non-toxic, binding domain. As this single unit was of low molecular polypeptide (~7.7 kDa), hence cross-linked rStxB was used. Results indicated that the heterologous expression of StxB was a suitable alternative for obtaining Stx and producing high-titre specific antibodies. Cross-linking of StxB with glutaraldehyde resulted in improvement of immunogenicity as revealed by production of high-titre antibodies in both the animals. Antibodies raised against StxB were used for its specific detection by sandwich ELISA, dot-ELISA and flow-through assay. In a study, production of STEC antiserum and the generation of a simple, cost-effective, sensitive and specific latex agglutination assay for establishing an aetiological diagnosis of STEC was developed13. Various concentrations of purified Stx were tested by the sandwich ELISA to evaluate its sensitivity for the detection of purified Stx14. Bioassay using Vero cells for the detection of Stx has been reported; however, it requires cell culture facilities and is expensive, time consuming and labour intensive and cannot be carried out in field. Though colony blot assay has been reported by Strockbine et al15, simpler immunological test can be a better substitute. The development of colorimetric lateral flow assay has also been reported which detects Stx within 10 min. The researchers have used a pair of monoclonal antibodies that bind epitopes which are common to Stx1 and six Stx2 variants16. In the present study, we have used highly specific polyclonal antibodies to develop cost-effective and field-amenable detection assays without requiring skilled manpower and instrumentation. The flow-through assay proved to be rapid as it detected Stx in five minutes in the field. The methodology used in our study may prove its potential for the detection of other toxins and biological agents.
Acknowledgment:
The authors thank the Director DRDE, for the support in this work.
Financial support & sponsorship: All the experimentation was done from in house funding, no external funding sought for the present work.
Conflicts of Interest: None.
References
- Shiga toxin and tetanus toxin as a potential biologic weapon. Pol Merkur Lekarski. 2015;39:157-61.
- [Google Scholar]
- Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403:669-72.
- [Google Scholar]
- Agar underlay method for recovery of sublethally heat-injured bacteria. Appl Environ Microbiol. 1999;65:5334-7.
- [Google Scholar]
- Multi-gene amplification: Simultaneous detection of three virulence genes in diarrhoeal stool. Mol Microbiol. 1989;3:1729-34.
- [Google Scholar]
- Laboratory diagnosis of bacterial diarrhea. In: Rubin SJ, ed. Coordinating: American society for microbiology. Washington, D.C: Cumitech; 1980. p. :12.
- [Google Scholar]
- Interaction of Shigella Shigae cytotoxin with receptors on sensitive and insensitive cells. J Recept Res. 1980;1:199-213.
- [Google Scholar]
- Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980;12:361-6.
- [Google Scholar]
- Isolation and characterization of Shigella Shigae cytotoxin. J Biol Chem. 1980;255:284-9.
- [Google Scholar]
- Rapid serological profiling by enzyme-linked immunosorbent assay. I. Measurement of antibody activity titer against Newcastle disease virus in a single serum dilution. Avian Dis. 1983;27:161-70.
- [Google Scholar]
- Recombinant Shiga toxin B subunit elicits protection against Shiga toxin via mixed Th type immune response in mice. Vaccine. 2011;29:8094-100.
- [Google Scholar]
- Antibodies against recombinant Shiga toxin subunit B neutralize Shiga toxin toxicity in HeLa cells. Protein Pept Lett. 2010;17:774-81.
- [Google Scholar]
- The cytotoxic activity of Shigella toxin. Evidence for catalytic inactivation of the 60 S ribosomal subunit. J Biol Chem. 1981;256:8739-44.
- [Google Scholar]
- Development of a simple latex agglutination assay for detection of Shiga toxin-producing Escherichia coli (STEC) by using polyclonal antibody against STEC. Clin Vaccine Immunol. 2007;14:600-4.
- [Google Scholar]
- Enzyme-linked immunosorbent assay to detect Shiga toxin of Shigella dysenteriae and related toxins. J Clin Microbiol. 1987;25:115-8.
- [Google Scholar]
- Characterization of monoclonal antibodies against Shiga-like toxin from Escherichia coli. Infect Immun. 1985;50:695-700.
- [Google Scholar]






