Translate this page into:
Seroprevalence of measles, mumps & rubella antibodies among 5-10 years old children in north India
For correspondence: Dr Madhu Gupta, Professor, Department of Community Medicine and School of Public Health, Postgraduate Institute of Medical Education & Research, Sector 12, Chandigarh 160 012, India e-mail: madhugupta21@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Globally, there is an effort to eliminate the measles and control rubella as these diseases lead to considerable morbidity and mortality especially among under-five children and are important public health problems. This study was aimed to estimate the seroprevalence of measles, mumps and rubella (MMR) antibodies among children of age 5-10 yr in Chandigarh, north India, to provide evidence on prevalent immunity levels.
Methods:
This cross-sectional study was conducted in Chandigarh, among 196 randomly selected healthy children (5-10 yr), who received either one or two doses of measles or MMR combination vaccine. Socio-economic background and immunization history were recorded. Blood sample (2 ml) was collected to estimate the MMR IgG antibody titres by using ELISA kits.
Results:
Protective seroprevalence of MMR antibodies was 40.8, 75.5 and 86.2 per cent, respectively. The geometric mean titres of MMR IgG antibodies in the study children were 11.3, 50.6 and 54.3 international units (IU)/ ml, respectively. The proportion of seroprotected children for measles was significantly higher among those who had received two or more doses (46.4%) of measles vaccine compared to those who had received single dose (35.6%) (P<0.001). About 16 per cent of children had received single dose of MMR vaccine. Among these, 71.4 and 100 per cent were seroprotected against mumps and rubella, respectively.
Interpretation & conclusions:
A large proportion of children aged 5-10 yr lacked protective immunity against measles (60%); about one-fourth (15-25%) were susceptible to infection with mumps and rubella virus. Mumps vaccination may be considered to be included in National Immunization Schedule for children with periodic serosurveillance.
Keywords
Antibodies
measles
mumps
rubella
seroprevalence
seroprotection
Measles is a highly infectious communicable disease of children characterized by fever with generalized body rash and complications such as pneumonia, ear infections, diarrhoea and subacute sclerosing pan-encephalitis, which can prove fatal. The median case fatality ratio of measles was 1.5 per cent in community based settings and 2.9 per cent in hospital based settings1. The incidence of measles in India was estimated to be 19 cases per million population for 2015, with estimated 49,200 deaths [95% confidence interval (CI): 35,400-65,500]2. Immunizing a child as per the National Immunization Schedule in India for measles i.e., first dose at the completion of nine months and second dose at 16-24 months of age, can prevent the occurrence and severity of this disease3. However, coverage of measles vaccine (89.1%) is less as compared to other vaccines such as BCG (91.9%) in India, which renders a number of children susceptible to this disease and acts as a potential source for measles outbreak4. Although rubella and mumps infection among children are milder diseases, there are several outbreaks of these two diseases reported among vulnerable population in India5. Rubella outbreaks mimic measles outbreak among children6. Infection with rubella during pregnancy can lead to congenital rubella syndrome. Mumps can lead to infertility among males due to its complication of orchitis5.
Earlier, the Global Goal for Measles Control was to reduce measles deaths by 90 per cent by 2010 compared to the estimated number in 20007. Hence, the Government of India introduced second dose of measles vaccine to immunize all under-five children in May 20108. Subsequently, the 11 Member States of the World Health Organization South East Asian Region (WHO-SEAR) committed to eliminate measles and control rubella/congenital rubella syndrome by 20209. Therefore, the Indian government decided to provide measles rubella (MR) vaccine in a campaign mode to all children of age nine months to <15 yr and later replace the measles vaccine with MR in universal immunization programme in 201710. The Indian Academy of Paediatrics supported elimination of not only measles and rubella, but also of the mumps by administering two doses of measles, mumps and rubella (MMR) vaccine11. Since, there is evidence of waning immunity with time for these diseases12, it will be worthwhile to study the long-term antibody titres against these diseases in children.
This study was aimed to estimate the seroprevalence of MMR antibodies among children of age 5-10 yr in Chandigarh, north India, and to provide evidence on prevalent immunity levels among children of age 5-10 yr in the community against MMR.
Material & Methods
This cross-sectional study was conducted among children of age 5-10 yr in the catchment area of Civil Hospital, Chandigarh, India, which was the field practice area of department of Community Medicine, School of Public Health, Postgraduate Institute of Medical Education & Research (PGIMER), Chandigarh, from August 2014 to April 2015. It catered to a population of 71,106 (rural 58%, urban 42%). There were 5532 children in the age group of 5-10 yr, and coverage of the first dose of measles vaccine was 82 per cent, as per the Annual Health Survey Report, 2013-201413.
Sample size of the study was estimated to be 180, considering the seroprevalence of measles of 21 per cent14 after the first dose of measles vaccine, precision was 6.5 per cent, power was 80 per cent and non-response rate was 15 per cent. Healthy children between 5 and 10 yr of age residing in the study area were first listed, numbered and then randomly selected with the help of a computer-generated random number table. Children suffering from acute febrile illness, chronic diseases, immunodeficiency diseases such as HIV infection, or on corticosteroids, having a history of convulsions/epilepsy, received another live vaccine within the last four weeks, a history of administration of blood, plasma transfusion or immunoglobulin within the last three months or diagnosed with malignancy were excluded from the study.
Written assent from eligible children and informed consent from their parents were obtained prior to recruitment in the study. The Ethical Committee of PGIMER, Chandigarh approved this study. All the children who were found to be susceptible to the diseases were given vitamin A supplementation. Repeated measles vaccinations were not provided to these children as part of this study. However, these might have been covered under MR vaccination campaign.
Socio-economic status as per Brahm Govind Prasad socio-economic classification for 201615, age and sex of the child, area of residence and a history of immunization (cross-checked from immunization cards if available, or from mother and child tracking registers maintained by auxiliary nurse midwives of the study area) were recorded in a structured, pre-designed and pre-tested interview schedule. Blood sample (2 ml) was collected from each child by a trained nurse and was transported to the department of Virology on the same day. The serum was separated after centrifugation and samples were stored in −20°C in aliquots till tested. The MMR IgG antibody titres were estimated by using commercially available ELISA kit as per the manufacturer's instructions. Antibody level <8 international units per millilitre (IU/ml) was considered as negative, between 8 and 12 IU/ml as equivocal and >12 IU/ml as positive or protective for both measles (Demeditec, Measles IgG-ELSIA, Germany) and mumps (Demeditec, Mumps IgG-ELISA, Germany). Values <10 IU/ml were considered as negative, 10-15 IU/ml as equivocal and >15 IU/ml as positive for rubella (Nova Tec Immunodiagnostica, GmbH, Nova Lisa, Rubella IgG-ELSIA, Germany). Children having antibody levels above the cut-off for positive were considered as seroprotected.
Statistical analysis: Data were analysed using Statistical Package for the Social Sciences, version 16.0 (SPSS Inc., Chicago, IL, USA). Proportion of children with positive antibody titre levels for MMR was estimated for seroprevalence. Difference between two or more proportions was tested by Chi-square test. Geometric mean titres (GMTs) of the antibodies were estimated, and differences were compared using t test and ANOVA. Differences were considered significant at 95 per cent.
Results
A total of 196 children in the age group of 5-10 yr (mean age: 6.38±1.6 yr) were selected, of whom 51 per cent were males, 92.9 per cent belonged to rural area and 44.7 per cent belonged to Class IV socio-economic status, with a median per capita income of ₹1,625 (interquartile range ₹1,170-2,535) (Table I). Thirty five (17.9%) children had a history of fever with rash in the last one year. Antibody titres could not be estimated for measles, mumps and rubella in two (1%), five (2.6%) and nine (4.6%) cases, respectively, due to insufficient sample.
| Socio-demographic variables | Number of participants (%) |
|---|---|
| Age (yr) | |
| 5 | 87 (44.4) |
| 6 | 33 (16.8) |
| 7 | 32 (16.3) |
| 8 | 19 (9.7) |
| 9 | 9 (4.6) |
| 10 | 16 (8.2) |
| Gender | |
| Male | 100 (51.0) |
| Female | 96 (49.0) |
| Residence | |
| Rural | 182 (92.9) |
| Urban | 14 (7.1) |
| Socio-economic status15 | |
| Class I | 4 (2.0) |
| Class II | 20 (10.2) |
| Class III | 66 (33.7) |
| Class IV | 87 (44.4) |
| Class V | 19 (9.7) |
Overall, protective seroprevalence for MMR was 40.8, 75.5 and 86.2 per cent, respectively (Table II). The proportion of seroprotected children for measles was significantly higher among those who had received two doses (46.4%) of measles vaccine as compared to the single dose (35.6%) (P=0.011). About 28 (16.6%) children had received single dose of MMR vaccine. Among these, 20 (71.4%) and 28 (100%) were seroprotected against mumps and rubella, respectively.
| Vaccine dose administered | Negative N (%) | Equivocal N (%) | Seroprotected N (%) | P |
|---|---|---|---|---|
| Measles† | ||||
| 0 (n=10) | 9 (90.0) | 1 (10.0) | 0 | 0.011 |
| 1 (n=59) | 32 (54.2) | 5 (8.5) | 21 (35.6) | |
| >2 (n=127) | 45 (35.43) | 22 (17.3) | 59 (46.5) | |
| Total (n=196) | 86 (43.9) | 28 (14.3) | 80 (40.8) | |
| Mumps (MMR)† | ||||
| 0 (n=168) | 31 (18.5) | 5 (3.0) | 128 (76.2) | 0.951 |
| 1 (n=28) | 6 (21.4) | 1 (3.6) | 20 (71.4) | |
| Total (n=196) | 37 (18.9) | 6 (3.1) | 148 (75.5) | |
| Rubella (MMR)† | ||||
| 0 (n=168) | 10 (6.0) | 8 (4.8) | 141 (83.9) | 0.560 |
| 1 (n=28) | 0 | 0 | 28 (100.0) | |
| Total (n=196) | 10 (5.0) | 8 (4.1) | 169 (86.2) |
†Titres could not be estimated for measles, mumps and rubella antibodies in 2, 5 and 9 children, respectively
Non-significant relationship was observed between seroresponse rate against all the three diseases and age of the child (Table III). The median antibody titre levels against measles were higher in the group who had received two or more doses of measles vaccine as compared to single dose (Fig. 1). Children aged 5-6 yr had lower, 7 yr equal, and 8 yr had higher median antibody titre levels against mumps in the group that had received single dose of MMR vaccine as compared to the unimmunized group. The median antibody titre levels against rubella were higher among those who had received single dose of MMR vaccine as compared to the unimmunized group (Figs 2 and 3). Similar seroprotection rate was observed among younger (5-7 yr) and older (8-10 yr) children after measles/MMR vaccination (Table IV). The GMTs of MMR IgG antibodies in the study population were 11.3, 50.6 and 54.3 IU/ml, respectively (Table V). Female children had slightly higher GMT for measles as compared to males, but the reverse was observed for mumps (P<0.05).
| Age (yr) | Measles titres group† | Mumps titres group† | Rubella titres group† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Na, n (%) | Eb, n (%) | SPc, n (%) | Na, n (%) | Eb, n (%) | SPc, n (%) | Na, n (%) | Eb, n (%) | SPc, n (%) | |
| 5 (n=87) | 33 (37.9) | 15 (17.2) | 38 (43.7) | 18 (20.7) | 3 (3.4) | 64 (73.6) | 5 (5.7) | 5 (5.7) | 71 (81.6) |
| 6 (n=33) | 15 (45.5) | 4 (12.1) | 14 (42.4) | 7 (21.2) | 1 (3.0) | 24 (72.7) | 1 (3.0) | 0 | 32 (97.0) |
| 7 (n=32) | 14 (43.8) | 7 (21.9) | 11 (34.4) | 6 (18.8) | 1 (3.1) | 25 (78.1) | 0 | 1 (3.1) | 30 (93.8) |
| 8 (n=19) | 11 (57.9) | 1 (5.3) | 7 (36.8) | 2 (10.5) | 0 | 16 (84.2) | 4 (21.1) | 2 (10.5) | 12 (63.2) |
| 9 (n=9) | 4 (44.4) | 0 | 5 (55.6) | 1 (11.1) | 1 (11.1) | 6 (66.7) | 0 | 0 | 9 (100.0) |
| 10 (n=16) | 9 (56.2) | 1 (6.2) | 5 (31.2) | 3 (18.8) | 0 | 13 (81.2) | 0 | 0 | 15 (93.8) |
| †Total (n=196) | 86 (43.9) | 28 (14.3) | 80 (40.8) | 37 (18.9) | 6 (3.1) | 148 (75.5) | 10 (5.1) | 8 (4.1) | 169 (86.2) |
†Titres could not be estimated for measles, mumps and rubella antibodies in 2, 5 and 9 children, respectively. Na, no seroprotection; Eb, equivocal response; SPc, seroprotected
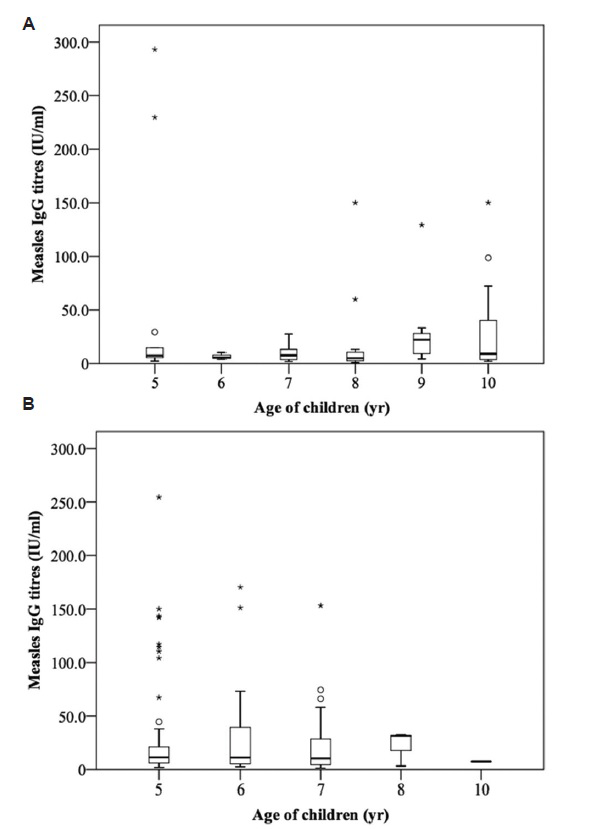
- Age-wise distribution of antibody titres against measles among children of age 5-10 yr who received single (A) or ≥two doses (B) of measles vaccine (*the outliers present beyond ±3 IQR; Osuspected outliers present between ±1.5 and 3.0 IQR). IQR, interquartile range.
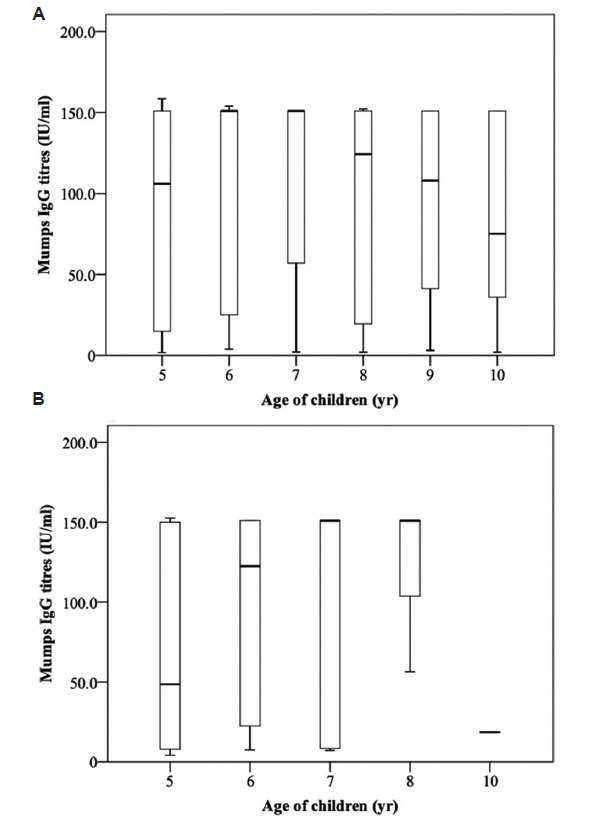
- Age-wise distribution of antibody titres against mumps among children of age 5-10 yr who were unimmunized (A) or received single (B) dose of MMR vaccine. MMR, measles, mumps and rubella.
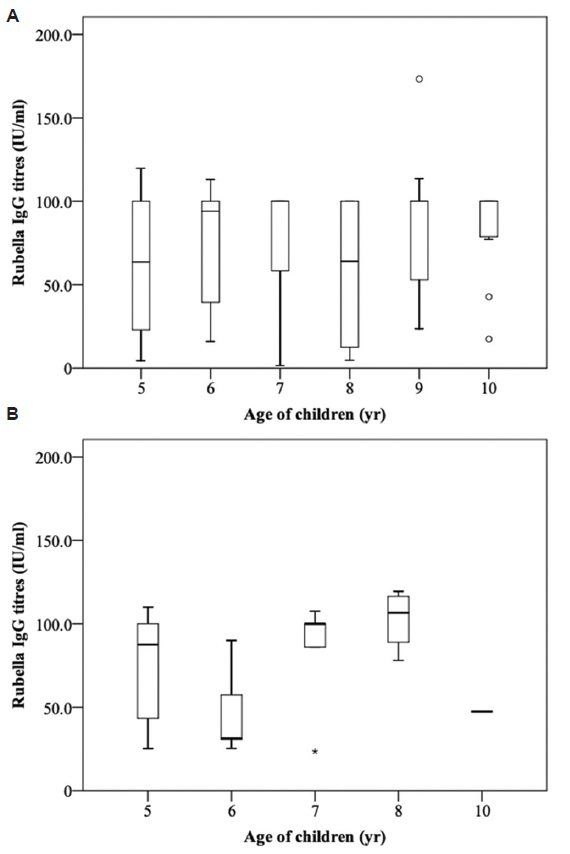
- Age-wise distribution of antibody titres against rubella among children of age 5-10 yr who were unimmunized (A) or received single dose (B) of MMR vaccine (*the outliers present beyond ±3 IQR; Osuspected outliers present between ±1.5 and 3.0 IQR). IQR, interquartile range; MMR, measles, mumps and rubella.
| Age (yr) | Immune response | Doses of measles | Doses of mumps (MMR) | Doses of rubella (MMR) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | ≥2 | Total | 0 | 1 | Total | 0 | 1 | Total | ||
| 5 | n | 2 | 13 | 72 | 87 | 74 | 13 | 87 | 74 | 13 | 87 |
| Seropositive, n (%) | 0 | 5 (38.5) | 33 (45.8) | 38 (43.6) | 55 (74.3) | 9 (69.2) | 64 (73.6) | 58 (78.4) | 13 (100) | 71 (81.6) | |
| 6 | n | 2 | 3 | 28 | 33 | 28 | 5 | 33 | 28 | 5 | 33 |
| Seropositive, n (%) | 0 | 0 | 14 (50.0) | 14 (42.4) | 20 (71.4) | 4 (80) | 24 (72.7) | 28 (100) | 5 (100) | 33 (100) | |
| 7 | n | 1 | 10 | 21 | 32 | 27 | 5 | 32 | 27 | 5 | 32 |
| Seropositive, n (%) | 0 | 3 (30) | 8 (38.10) | 11 (34.4) | 22 (81.4) | 3 (60) | 25 (78.1) | 25 (92.6) | 5 (100) | 30 (93.75) | |
| 8 | n | 2 | 12 | 5 | 19 | 15 | 4 | 19 | 15 | 4 | 19 |
| Seropositive, n (%) | 0 | 3 (25) | 4 (80.0) | 7 (36.8) | 13 (86.7) | 3 (75) | 16 (84.2) | 8 (53.3) | 4 (100) | 12 (63.15) | |
| 9 | n | 2 | 7 | 0 | 9 | 9 | 0 | 9 | 9 | 0 | 9 |
| Seropositive, n (%) | 0 | 5 (71.4) | 0 | 5 (55.5) | 6 (66.7) | 0 | 6 (66.7) | 9 (100) | 0 | 9 (100) | |
| 10 | n | 1 | 14 | 1 | 16 | 15 | 1 | 16 | 15 | 1 | 16 |
| Seropositive, n (%) | 0 | 5 (35.7) | 0 | 5 (31.2) | 12 (80.0) | 1 (100) | 13 (81.2) | 14 (93.3) | 1 (100) | 15 (93.75) | |
| Total | n | 10 | 59 | 127 | 196 | 168 | 28 | 196 | 168 | 28 | 196 |
| Seropositive, n (%) | 0 | 21 (35.6) | 59 (46.4) | 80 (40.8) | 128 (76.2) | 20 (71.4) | 148 (75.5) | 141 (83.9) | 28 (100) | 169 (86.2) | |
| P | - | 0.274 | 0.491 | 0.736 | 0.794 | 0.916 | 0.866 | 0.001 | - | 0.004 | |
| Groups (n) | Measles | Mumps | Rubella |
|---|---|---|---|
| GMT (95% CI) | GMT (95% CI) | GMT (95% CI) | |
| Gender | |||
| Male (100) | 10.13 (7.94-12.77) | 25.62 (13.73-44.21) | 36.23 (27.17-47.67) |
| Female (96) | 11.65 (9.04-15.11) | 15.04 (9.77-22.86) | 33.29 (25.17-43.41) |
| Age group (yr) | |||
| 5 (87) | 12.16 (9.43-16.08) | 16.56 (10.11-26.53) | 30.93 (23.52-41.18) |
| 6 (33) | 11.64 (7.80-17.67) | 38.95 (16.53-82.48) | 46.68 (34.79-64.83) |
| 7 (32) | 9.80 (6.58-14.87) | 11.93 (4.48-33.69) | 52.19 (27.76-81.58) |
| 8 (19) | 7.51 (4.60-12.37) | 7.99 (2.00-17.00) | 15.08 (12.00-22.00) |
| 9 (6) | 12.36 (6.07-28.05) | 12.60 (3.00-74.00) | 32.51 (24.00-5300) |
| 10 (16) | 8.77 (4.57-17.84) | 24.78 (9.08-69.32) | 36.21 (13.39-70.84) |
| Number of doses received | |||
| 0 | 3.05 (2.08-4.59) (n=10) | 17.44 (11.31-26.35) (n=168) | 30.49 (24.08-38.79) (n=168) |
| 1 | 9.23 (6.85-12.64) (n=59) | 21.64 (10.84-42.98) (n=28) | 54.07 (39.60-73.21) (n=28) |
| ≥2 | 12.94 (10.36-15.85) (n=127) | - | - |
CI, confidence interval
The proportion of seroprotected females (51%) against measles was not significantly higher as compared to males (49%), whereas for mumps and rubella, higher proportion of males were seroprotected (54.7 and 50.9%) as compared to females (45.3 and 49.1%), respectively but the difference was not significant. Seroprotection against measles was non-significantly higher in rural as compared to urban areas (40 vs. 35%). Proportion of seroprotected children against mumps and rubella was 100 per cent in urban areas. Among children who had a history of fever with rash (n=35), only a small proportion were seroprotected against measles (n=13, 37.1%), but majority of them were seroprotected against mumps (n=28, 80.0%) and rubella (n=31, 88.6%).
Discussion
The results of this study highlighted that there was a large proportion (60%) of children who did not have protective immunity against measles, and about 15-25 per cent of children were susceptible to infection with rubella and mumps viruses. This indicated a need to include mumps control plans in addition to measles elimination and rubella control strategic plans and implement these plans with increased intensity to achieve the WHO-SEAR goal by 20209.
The observation of higher seroprotection of children for measles among those who had received two doses of measles vaccine as compared to those who had received single dose or no dose in this study was similar to the findings of Sheikh et al16. Higher seroprevalence (44-76%) after single dose of measles vaccine than that observed in our study (36%) was documented from other developing countries1718. This indicates lower baseline immunity level for measles in these studies. The lower seroprotection for measles after two doses (21.4%) among 4 to 6 yr old children in a study by Gomber et al14, than that observed in this study (46.4%), was probably due to the immunosuppressant effect of maternal antibodies19.
Waning of MMR/measles vaccine-induced antibodies after second dose, and the possibility of secondary vaccine failure, as observed in this study, was in line with the existing literature18192021. Waning of both the concentration and the avidity of antibodies might contribute to measles and mumps infections and lower antibody levels in twice-MMR-vaccinated individuals12. Chen et al22 highlighted that the waning of vaccine-induced immunity to undetectable levels was more apparent in the Asian Population. An additional dose of measles antigen during the schoolgoing age or later to boost the individual as well as herd immunity against measles can be seen as a possible solution to counteract waning immunity. On the contrary, Yekta et al23 reported higher seropositivity and higher mean titres of measles antibody in children who received single vaccination as compared to those who were vaccinated twice against measles (P<0.05). This can be explained by the fact that pre-immunization antibody level is inversely correlated with the response to vaccination i.e., children with low pre-immunization antibody titres show strong response2024.
The results of this study showed that majority of unvaccinated children for mumps and rubella were seroprotected against mumps and rubella as has been reported earlier14. The reason for seroconversion (equivocal response) in one unvaccinated child for measles, who was eight yr old, could be due to the low-level circulation of the measles virus in the community. This indicates that community-acquired infections and the resultant natural immunity have a role to play in eliciting the immunological response against mumps and rubella. However, 15 per cent of children remain susceptible against rubella, which may pose a threat to infection in the community. Furthermore, it was seen that 100 per cent of the children who received even one dose of MMR in each age group were seroprotected against rubella, which justified the role of vaccination in this age group.
Higher seroprotection among females (44.8%) as compared to males (37%) observed in this study can be attributed to stronger humoral immune responses to measles vaccine by females, owing to the expression of several X chromosome-linked genes implicated in immunological processes2526. In another study27, a better rubella virus-specific antibody response was observed in males soon after vaccination, but no apparent gender difference was seen after 10 wk of vaccination, and girls were better seroprotected in the later stages of life. Higher seroprotection against measles in children in rural areas could be attributed to higher levels of virus transmission because of a large number of migrant population residing in the urbanized, overcrowded rural area in the study setting. Major differences with regard to the immunization coverage were not expected between rural and urban populations within Chandigarh28. Higher mumps and rubella seroprevalence in urban areas could be due to more access to MMR vaccine due to better awareness and education levels29. In the present study, no significant association was seen between the socio-economic status and seroprevalence rate of MMR. Lower socio-economic status of the parents might affect the vaccine coverage, but had little effect on the seroconversion, as was also reported by Wright et al and Polack30. Another observation that higher (88.6%, 31/35) cases with a history of fever with rash were seroprotected against rubella as compared to measles (37.1%, 13/35) could be because of previous illness by either rubella or other exanthematous illness and not specifically measles.
The strength of this study was its community-based study design that provided baseline seroprevalence data of MMR. The limitations were that antibody titres could not be estimated in 16 (8%) children due to insufficient blood sample. However, a non-response rate of 15 per cent was considered while estimating the sample size. The nutritional assessment of the children was also not done.
The public health importance of this study was that it provided evidence of low seroprevalence of measles among school going children, which might have implications in terms of achieving the goal of elimination of measles in WHO-SEAR. Low seroprevalence of measles also indicated the need to monitor routine immunization sessions, especially vaccine storage and cold chain maintenance, which may lead to low serologic responses18. The results also highlight the need to incorporate mumps vaccine along with measles and rubella vaccine in the National Immunization Schedule as the study population was not immune to mumps, to introduce MMR booster dose at 4-6 yr31 and to conduct periodic serosurveillance for MMR so that elimination/control goal could be achieved.
Acknowledgment:
Authors thank Dr Krishna Chaudhary, Senior Medical Officer, Civil Hospital, Chandigarh, for cooperation; Shri Chering Bhag, Medical Social Worker, Department of Community Medicine, PGIMER, for data collection; and Ms Jasjeet Kaur, Laboratory Technician, department of Virology, PGIMER, for ELISA testing.
Financial support & sponsorship: This study was financially supported by the Department of Science and Technology, Chandigarh (Ref. No. S&T/Sanc/06/2014/456-462).
Conflicts of Interest: None.
References
- Estimates of case-fatality ratios of measles in low-income and middle-income countries: a systematic review and modelling analysis. Lancet Glob Health. 2019;7:e472-81.
- [Google Scholar]
- Progress towards regional measles elimination-Worldwide, 2000-2015. WHO Wkly Epidemiol Rec (Geneva, Switzerland). 2016;91:525-36.
- [Google Scholar]
- Ministry of Health & Family Welfare. Universal Immunisation Programme. New Delhi: MoHFW, Government of India; Available from: https://www.nhp.gov.in/sites/default/files/pdf/Immunization_uip.pdf
- International Institute of Population Sciences. National Family Health Survey (NFHS-4). India Fact Sheet. 2015-16. New Delhi: Ministry of Health & Family Welfare, Government of India; Available from: http://www.rchiips.org/NFHS/pdf/NFHS4/India.pdf
- [Google Scholar]
- Introducing combined measles, mumps and rubella vaccine in Chandigarh, India: Issues and concerns. Indian Pediatr. 2014;51:441-3.
- [Google Scholar]
- An outbreak of rubella in a union territory of Northern India. Indian Pediatr. 2014;51:897-99.
- [Google Scholar]
- Measles vaccine: WHO position paper. WHO Wkly Epidemiol Rec (Geneva, Switzerland). 2009;84:349-60.
- [Google Scholar]
- Introduction strategy of a second dose measles containing vaccine in India. Indian Pediatr. 2011;48:379-82.
- [Google Scholar]
- Strategic plan for measles elimination and rubella and congenital rubella syndrome control in the South-East Asia Region, 2014-2020. WHO, Regional Office for South East Asia. Available from: http://www.searo.who.int/entity/immunization/documents/sear_mr_strategic_plan_2014_2020.pdf
- [Google Scholar]
- IAP position paper on burden of mumps in India and vaccination strategies. Indian Pediatr. 2015;52:505-14.
- [Google Scholar]
- Waning antibody levels and avidity: Implications for MMR vaccine-induced protection. J Infect Dis. 2012;206:1542-8.
- [Google Scholar]
- Department of Community Medicine and School of Public Health. In: Annual Health Survey Report-2013-14. Chandigarh: Postgraduate Institute of Medical Education & Research;
- [Google Scholar]
- Immune response to second dose of MMR vaccine in Indian children. Indian J Med Res. 2011;134:302-6.
- [Google Scholar]
- Online interactive calculator for real-time update of the Prasad's social classification. Available from: www.prasadscaleupdate.weebly.com
- [Google Scholar]
- A comparison study of measles antibody between two doses vaccination at 9, 18 months and single dose at 9 months in children 4-6 years old. J Med Assoc Thai. 2011;94:309-15.
- [Google Scholar]
- Immune response to 1 and 2 dose regimens of measles vaccine in Pakistani children. Hum Vaccin Immunother. 2013;9:2529-32.
- [Google Scholar]
- IgG avidity to distinguish secondary from primary measles vaccination failures: Prospects for a more effective global measles elimination strategy. Expert Opin Pharmacother. 2003;4:1215-25.
- [Google Scholar]
- Waning immunity and subclinical measles infections in England. Vaccine. 2004;22:4110-6.
- [Google Scholar]
- Duration of immunity following immunization with live measles vaccine: 15 years of observation in Zhejiang province, China. Bull World Health Organ. 1991;69:415-23.
- [Google Scholar]
- Immune response to measles vaccine after mass vaccination in Urmia, Islamic republic of Iran. East Mediterr Health J. 2009;15:516-25.
- [Google Scholar]
- The effect of measles-mumps-rubella (MMR) immunization on the immune responses of previously immunized primary school children. Vaccine. 2003;21:2580-8.
- [Google Scholar]
- Seroprevalence of measles-, mumps- and rubella-specific IgG antibodies in German children and adolescents and predictors for seronegativity. PLoS One. 2012;7:e42867.
- [Google Scholar]
- How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. 2015;14:309-21.
- [Google Scholar]
- Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109:9-15.
- [Google Scholar]
- International Institute for Population Sciences. In: District level household and facility survey (DLHS-4), 2012-13. India, Mumbai: IIPS;
- [Google Scholar]
- Inequity in childhood immunization between urban and rural areas of Peshawar. J Ayub Med Coll Abbottabad. 2011;23:134-7.
- [Google Scholar]
- Understanding variation in measles-mumps-rubella immunization coverage - A population-based study. Eur J Public Health. 2006;16:137-42.
- [Google Scholar]
- Centers for Disease Control and Prevention. Recommended immunization schedule for children and adolescents aged 18 years or younger, United States. 2017. Atlanta: CDC; Available from: https://www.cdc.gov/vaccines/schedules/hcp/imz/child-adolescent-shell.html#f8 2017
- [Google Scholar]






