Translate this page into:
Redox regulation & sperm function: A proteomic insight
For correspondence: Dr Gayatri Mohanty, Redox Biology Laboratory, Department of Zoology, Ravenshaw University, Cuttack 753 003, Odisha, India e-mail: gayatri_mohanty32@yahoo.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Infertility affects nearly 15 per cent of all couples within the reproductive age worldwide, with about 50 per cent being exhibited in the male, called male factor infertility. Successful reproduction is dependent on sperm chromatin integrity. Spermatozoa are highly specialized cells that aim to transmit the paternal genomic blueprint to the oocyte. The spermatozoon is regulated by redox mechanisms during its epididymal transit to acquire fertilizing ability. While, at physiological levels, the production of reactive oxygen species (ROS) supports the spermatozoon to acquire its fertilizing ability, at high concentrations, it affects sperm function leading to infertility. Emerging proteomic technologies provide an opportunity to address these key issues that may solve many fertility-associated problems resulting from oxidative stress (OS). This review highlights the need for an efficient therapeutic approach to male infertility with the application of high-throughput OS-mediated proteomic technology, and also addresses the question as to whether targeting these altered sperm-specific proteins may help in designing an efficient and reversible male contraceptive.
Keywords
Male contraception
male infertility
proteomics
reactive oxygen species
Introduction
Reproductive success is essential for the health of individuals, but as the world's population continues to rise, the need for an effective contraceptive method has become increasingly important. While coitus interruptus, prolonged lactation forms of contraceptive methods have been in use since ancient times, there has been a remarkable increase in the use of modern contraceptive methods in both developed and developing countries1. Ironically, the need for a better contraceptive option is still warranted2. This makes it pertinent to have a closer look at the choices available in the market with regards to contraceptive options. Although female contraceptive methods have gained much attention which have been developed and widely marketed, still, the degree of their efficacy is often questionable. In contrast, male contraception has been restricted to condoms, which pose logistical challenges, and vasectomy, that is largely irreversible2, making the contraceptive choices for men limited3. Male contraceptive methods account for only 14 per cent of those used worldwide and no pharmaceutical male contraceptive methods is available so far4.
Infertility is a condition that affects nearly 15 per cent of all couples within the reproductive age, wherein about 50 per cent is known to be associated with the male resulting in male factor infertility. However, it is known that a multitude of factors may lead to male factor infertility, but still, in majority of cases, its cause remains largely idiopathic5678. The most desirable features of an efficient male contraceptive are simplicity, reversibility and effectiveness. However, with the non-availability of an efficient male contraceptive method, a closer look in understanding molecular mechanisms of male infertility is an important aspect that needs to be investigated. This is not only important for the treatment of infertility but also for providing clues to target involved biomolecules for the design of contraceptives. This has opened new avenues for research that include studies on genes, proteins and enzymes associated with the male reproductive system9. It has been realized that proteins - the final product of genes, critically affect the phenotype. At the reproductive level, these new methods are targeted to specific interactions between the male and female gonads as well as between spermatozoa and ova10111213. Therefore, the field of sperm proteomics takes the centre stage for the understanding of male fertility potential. The state of oxidative stress (OS) is detected in about 25-80 per cent of infertile men and is known to be critically involved in the pathology of male infertility14. Proteins are known to undergo easy modulation by reactive oxygen species (ROS). This close interaction between the proteins and high levels of ROS makes it pertinent to understand and examine its impact on sperm proteomic profile in different pathological states. Thus, this futuristic approach aims at targeting the dysregulated proteins that affect the redox signalling mechanism in male infertility. The present review highlights the need for an efficient therapeutic approach to male infertility with the application of high-throughput OS-mediated proteomic technology and whether manipulations at the targeted proteins may help to find an effective and reliable solution for the development of a suitable male contraceptive.
Oxidative stress and sperm function
Semen quality has often been used as an indirect measure of male infertility regardless of its utility been raised many times in the current use of assisted reproductive technology. This includes examination of sperm count, motility and morphology that have been commonly used in fertility clinics as a part of routine semen analyses15. However, with its limited degree of prognostic and diagnostic information, the role of semen analysis has not only been challenged but has boosted the search for new and alternate methods. The assessment of male fertility potential has thus shifted its focus towards sperm DNA damage in the management of male infertility1617. However, despite the numerous assays that have been established in relation to sperm DNA damage our understanding still remains incomplete with regards to its aetiology, measurement and clinical implications. It is necessary to develop a standardized protocol and substantiate the exact contributions of sperm DNA damage within the myriad of other male and female factors resulting in undesirable reproductive outcome1718.
The above-mentioned facts have made it important to delve into other factors that lead to the decline in human semen quality. There have been several explanations to this phenomena that include environmental stress, modern lifestyle, infection and toxic chemicals that may alter the endocrine system resulting in steady decline of the male reproductive potential. This necessitates to have a closer look on free radicals and ROS since these regulate several key events pre- and post-fertilization1319. OS in a cell is not only characterized by an overproduction of ROS but also through the loss of antioxidative capacity present in the cell, and is therefore considered as one of the leading causes for male infertility20. ROS are considered to be highly reactive oxidizing molecules, with the presence of unpaired electrons. OS has the ability to cause alterations at the molecular level, that has an adverse impact on lipids, proteins, and DNA2122. This array of cyclic redox mechanism not only impairs sperm function but also leads to perturbations in semen parameters and eventual infertility.
Sperm proteomics
The testis provides an environment for efficient sperm production through the process known as spermatogenesis23. The process of spermatogenesis constitutes highly complex cellular changes that include the proliferative, the meiotic and the differentiation phases leading to the production of a matured spermatozoon. Mature spermatozoa are highly differentiated cells with a silent transcriptional and translational machinery. This implies that studies on transcriptomics and genomics are less informative24. On the other hand, in vitro studies using sperm culture techniques for a long duration is difficult to study gene function that regulates sperm function. In this context, several molecules have been identified, including mRNAs, non-coding RNAs and proteins2526. Specific alterations at the sperm proteomic levels have been identified to be the cause of perturbations within the spermatozoa, and several protein identification strategies have been utilized to substantiate the said fact. High-throughput proteomic technologies have emerged as a powerful tool to study and analyze the role played by different sperm proteins to execute its function. Target-specific studies are aimed mainly towards the quantification and identification of one or a few key proteins through the use of specific antibodies as well as several biochemical assays27282930. Wider proteomic technology is dependent on mass spectrometry (MS) analysis to identify sperm proteomic profile on a large scale3132333435. Till date, the utility of MS analysis in the field of sperm proteomics has led to the identification of thousands of proteins known to be associated with the testis as well as with the mature spermatozoon. The array of information thus collected has been useful in understanding of sperm biology at a deeper level11. However, defective sperm maturation process may be considered as one of the important attributors for a steady decline in semen parameters as observed in infertile men. Thus, it becomes significant to focus on specific proteins that are known to have a key role in sperm function and when altered may lead to several male factor anomalies. In this context, research on the proteomic profile on mature and immature spermatozoa seems important wherein ejaculated spermatozoa collected from healthy fertile donors were categorized into four fractions using three-layer density gradient. This study7 highlighted the increased detection of proteins associated with sperm function and metabolic processes such as cell motility, energy metabolism and oxidative phosphorylation processes. While a decreased detection of proteins associated with transcriptional and translational machinery, and response to OS processes was observed7. This ideology has further raised the willingness to compare and examine the sperm samples with defined aetiologies through differential proteomic technology which aims to compare sperm samples3637383940.
Male infertility and sperm proteomics
Sperm motility and fertilization ability are recognized as key regulators of male fertility potential, and alterations have been observed in about 25 per cent of male infertility cases varying from varicocele to obesity or smoking habits41. However, there exist several discrepancies when sperm parameters are taken into consideration for the diagnosis and assessment of male fertility potential. In one of the reports41, the two-dimensional (2D) proteomic profiling in a patient with failed in vitro fertilization revealed 20 proteins to be differentially expressed as compared with controls. Subsequently, differential protein expression profiles associated with several disease condition (asthenozoospermic, oligozoospermic and teratozoospermic) have also been identified. 2D-fluorescence difference in gel electrophoresis (DIGE) proteomic technology has been applied for the analysis of globozoospermic condition, resulting in the identification of key proteins to be differentially expressed. Nine proteins were found to be upregulated while 26 proteins were downregulated as compared their normal counterparts42. To date, several proteomic studies have been conducted based on defined aetiologies, and several key proteins present in different component of spermatozoa has been identified as has been enlisted13. In a proteomic study conducted on infertile men affected with varicocele, a total of 99 proteins were found to exhibit a significant difference in expression levels41. The study further identified and validated key proteins (PKAR1A, AK7, CCT6B, HSPA2 and ODF2) known to have a role in stress response and sperm function.
Proteins present in the seminal plasma may contain important information regarding testicular function, especially because around 10 per cent of the proteins found in seminal plasma arise from the testes43. A comparative proteomic study on seminal plasma between fertile and post-vasectomy men revealed many key proteins unique or at an elevated level with respect to the other. This study concluded that these proteins had their origin at the testicular or epididymal level and might be of clinical relevance in diagnosis and assessment of male infertility as well as other urogenital diseases44. Secretions from several male accessory glands that include prostate, seminal vesicles, epididymis, and Cowper's gland constitute the seminal plasma with a protein concentration that ranges from 35 to 55 g/l45. The comparative proteomic study using 2D PAGE and tandem MS was undertaken on seminal plasma of patients that exhibited azoospermia and vasectomy condition. The study identified several protein spots in the seminal plasma under the diseased condition to be differentially expressed46. On the contrary, a study revealed no significant difference in proteins in the seminal plasma of normozoospermic, oligozoospermic and asthenozoospermic groups with the exception of azoospermic condition utilizing 2D DIGE/MS approach47. The focus of our study48 was to identify and investigate the status of seminal plasma proteins in several male pathophysiological states which included teratozoospermic and oligozoospermic conditions as compared to their normal ones. The study identified several proteins to be differentially regulated. Bioinformatic analysis of these proteins revealed the identification of a majority of proteins in the extracellular region with biological regulation as the major affected process48. Research focussing at sperm and seminal plasma proteomics is ongoing, and different researchers have proposed different sets of proteins as biomarkers in different aetiopathological conditions. Application of high-throughput proteomics technologies has resulted in minimal overlap between independent studies and has evolved as one of the major shortcomings of sperm proteomic approach49. This variability in data has forced researchers to go to a step further by evaluating sperm proteins mediated through redox regulation that holds a great promise on more reproducible results in the future.
Reactive oxygen species-mediated male infertility and proteomics technology
ROS production at physiological levels is required for the maintenance of normal sperm function while its overproduction is detrimental to sperm survival and function. Although the exact mode of action of OS-induced decline in sperm function remains largely unknown, but some of the existing key attributors for deteriorating sperm function include axonemal damage through peroxidation, loss of intracellular ATP levels, followed by the production of 4-hydroxynonenal and malondialdehyde as a result of lipid peroxidation50. Sperm proteins are known to predominantly involve in sperm function and OS-induced sperm damage may closely interact with these proteins. This may lead to alterations in protein expression levels which may contribute to abnormal sperm morphology, poor sperm motility and fertilization failures. Sharma et al51 conducted a study on the proteomic profile of spermatozoa proteins with OS as the centre stage. The study evaluated the proteomic profile of male infertile patients by classifying the seminal ejaculates into high and low levels of ROS (denoted by ROS+ and ROS−). Intriguingly, the study identified several key proteins with altered expression (10 proteins to be overexpressed and 5 proteins to be underexpressed with a 2-fold difference) in the ROS+ group. Further, bioinformatic analysis revealed the major involvement of these key proteins towards response and management of OS51. Thus, high-throughput proteomic techniques used for investigating and analyzing the human spermatozoon has opened new avenues for research in unravelling the underlying aetiology for male infertility. Since the observed proteomic changes within the spermatozoon could be a probable factor in the disruption of sperm physiological process and function, studies were conducted to evaluate the differential protein expression levels in spermatozoa from patients who exhibited high, low and medium ROS levels. With the application of LTQ-Orbitrap elite hybrid MS analysis, the study revealed six proteins with distinct reproductive functions to be differentially expressed in all three ROS groups while being absent in the control14.
Seminal plasma being a natural reservoir of antioxidants is responsible for overall nourishment and protection of the spermatozoa within the female reproductive tract resulting in successful fertilization and implantation of the embryo. A study was conducted to identify the differentially expressed proteins in the seminal plasma of men with high and low levels of ROS (denoted as ROS+ and ROS− groups)52. The proteomic profiling which showed an upregulation of proteins in the ROS+ group included prolactin induced protein (PIP), semenogelin II (SEMG2) precursor and acid phospatase, protrate (ACPP) short isoform precursor. The downregulated ones included the clusterin (CLU) preprotein, zinc-alpha-2 glycoprotein 1 (AZGP1), prostate specific antigen (KLK3) isoform 4 preprotein and semenogelin1 isoform a preprotein (SEMG1). Further, gene ontology mapping revealed a high degree of distribution of proteins in the extracellular region with a major role in antioxidative activity and regulatory processes. The study concluded that men exhibiting high levels of ROS in their seminal ejaculate mostly exhibited altered protein expression levels (upregulated or downregulated) and could therefore, potentially contribute to male infertility. Further, as a step forward comparative proteomic analysis of seminal plasma proteins with varying levels of ROS from fertile and infertile men was undertaken. The main focus was identification of signature proteins that were associated in ROS-mediated reproductive dysfunction53. The study showed that membrane metalloendopeptidase and FAM3D along with concomitant assessment of ROS levels in the seminal plasma might serve as potential biomarkers for the diagnosis of male infertility.
Redox regulation: An insight into sperm chromatin dynamics
The process of spermatogenesis comprises differentiation of primordial germ cells into spermatogonia, followed by the production of primary and secondary spermatocytes, spermatids, and finally, a mature spermatozoon capable of fertilizing the oocyte54. Spermiogenesis that constitutes differentiation of round haploid spermatids into mature spermatozoon plays a pivotal role in the aetiology of DNA damage in the male germ line. This involves extensive remodelling of the sperm chromatin with the sequential replacement of histones by the highly positively charged protamines so as to lodge the entire haploid genome into a 52.5 mm sperm head55. This extensive packaging of sperm chromatin occurs during its epididymal transit which involves two important events. The first step constitutes the introduction of DNA strand breaks by topoisomerases to relieve torsional stress, which is corrected by a complex process H2Ax phosphorylation, formation of poly ADPribose (PAR) by nuclear PAR polymerases and topoisomerase56. In the second step, the sequential replacement of histones by protamines is promoted by intermediary transition proteins and is thought to play a key role in maintaining DNA integrity. The most striking difference in amino acids that exists between histones is Cys residues. While histones exhibit the presence of only one Cys residue, it is found in abundance in the protamines of mammalian spermatozoon. This Cys residue is the main target for disulphydryl formation. High sperm viability is contributed by the regulated disulphydryl bond formation in proteins and any alteration may lead to sperm dysfunction and may be a probable cause for male infertility. Many proteins are not destroyed by the damaging effects of OS but rather undergo ROS-mediated thiol modifications which modulate specific protein function. This concept has led researchers to further gain deeper knowledge on the contributions from a defective paternal genome post-fertilization and during early embryogenesis. Study conducted by our group on seminal ejaculate in couples affected by idiopathic recurrent pregnancy loss (iRPL) highlighted the idea as to whether there exists any significant relationship between increased retention of histones due to defective sperm chromatin packaging and OS57. The study revealed that predominance of OS levels in terms of protein carbonylation and lipid peroxidation existed concomitant with an excess retention of histones in the semen profile of iRPL groups as compared to their normal counterparts. The study concluded that histones which are rich in arginine and lysine residues were easy targets for oxidative modification and might be responsible for disturbing the paternal epigenomic control during early embryogenesis leading to early abortion. Thus, it may be concluded that oxidative modifications generally occur on Cys residues.
Male epigenome and developing embryo: Altered redox regulation
Studies on sperm chromatin have revealed the presence of regulatory elements that have the ability to regulate gene silencing or activation upon delivery to the egg58. It has also been shown that the fertilizing spermatozoa not only deliver its haploid genome but also epigenome, which contains the essential code necessary for reprogramming during zygote formation59. About 95 per cent of the human sperm chromatin is formed by highly compact toroidal nucleoprotamine complexes while the remaining 5-10 per cent is organized with histones. Of particular importance is the retention of these non-canonical histones at specific loci. Thus, it has been observed that these non-random distributions of genes, gene sequences and repetitive elements have the potential to be involved in the sperm chromatin reorganization in the oocyte and perhaps in the selective activation of key paternal genes in the early embryo60. Our study57 on degree of retention of histones through aniline blue staining in RPL patients as compared to their normal counterparts revealed a greater proportion of intermediately stained spermatozoa in the RPL group as compared to the lightly stained spermatozoa in the control group. This indicates that there has been epigenetic modifications during sperm chromatin remodelling with insufficient histone-protamine replacement that though promotes fertilization but disrupts early embryogenesis. The findings were further correlated with several OS parameters in both sperm and seminal plasma which revealed oxidative predominance in terms of thiol redox status, protein carbonylation in association with lipid peroxidation in the experimental group57.
Redox proteomics: Key to male fertility potential
Soon after its testicular release, the immature spermatid undergoes a series of dynamic modifications during its epididymal transit so as to become terminally differentiated spermatozoa capable of fertilizing the ovum. Redox proteome denotes that components of the proteome that have the ability to undergo potentially reversible redox reactions are easy targets for reactive species during OS that undergo irreversible modifications61. Thus, redox proteomics is aimed at the identification and quantification of redox-mediated changes within the proteome both in redox signalling and under OS conditions. During their epididymal transit, spermatozoa undergo several maturational changes irrespective of their silenced transcriptional and translational machinery. This dynamic modifications within the spermatozoa are brought about either by the acquisition of new proteins through epididymosomes or by several post-translational modifications expressed on histone tails1262. Histone modification is one of the remarkable events during chromatin remodelling that represents an important epigenetic chromatin marks critical for early embryogenesis. Methylation, acetylation, phosphorylation, ubiquitination, ribosylation and sumoylation are some of the post-translational modifications that occur on histone tails. Histone marks are a dynamic process since histone modifications can be easily induced and removed by a wide range of enzymes. The introduction of these post-translational modifications can occur as a result of attack by ROS and reactive nitrogen species (RNS) on proteins. These redox reactions have a central role in establishing homeostasis between the epigenome, epiproteome and external environment. It is hypothesized that the changes in oxidative proteins may be manipulated to find an effective solution to male infertility (Figure).
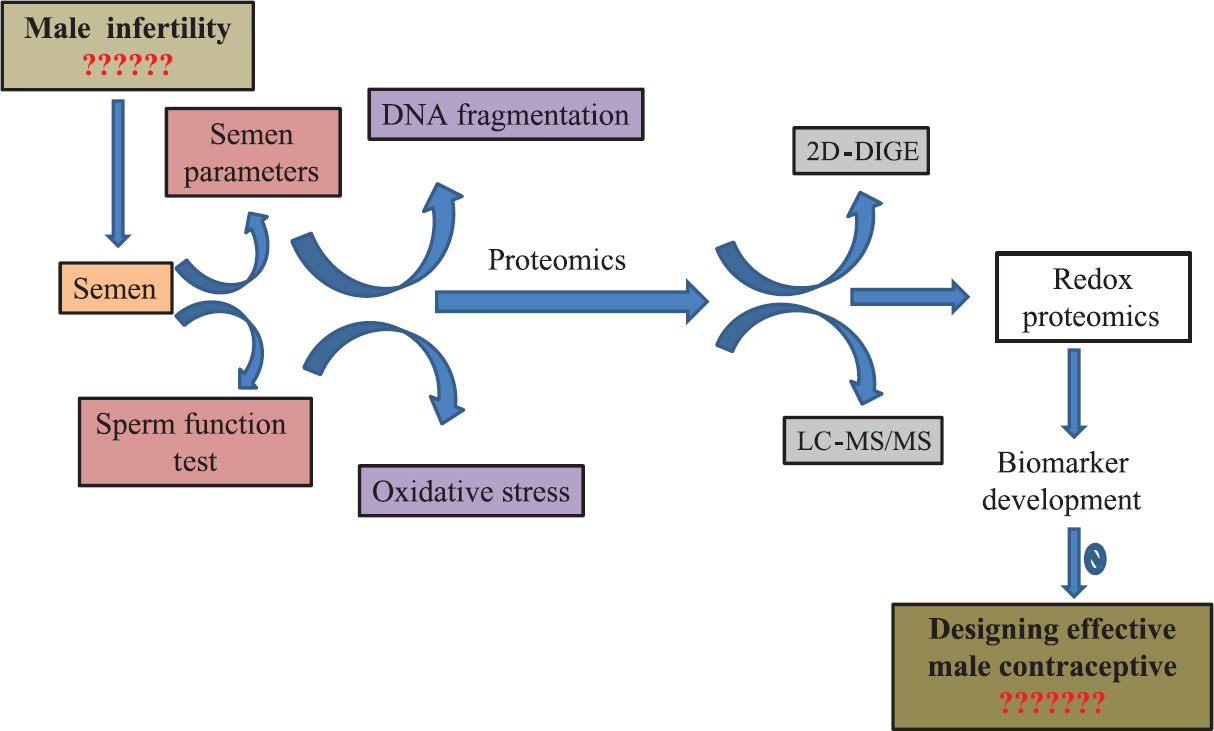
- Schematic representation of progression in sperm functional analysis in understanding male fertility potential with a scope for developing suitable biomarkers and their manipulations to enable designing an effective male contraceptive. 2D, two-dimensional; DIGE, differential gel electrophoresis; LC-MS/MS, liquid chromatography-tandem mass spectrometry.
Reproductive health status and its future
Improved tools for the diagnosis and prognosis of male fertility potential would put the clinicians at an advantageous position to treat couples seeking help for a variety of infertility associated problems. Perturbations in redox homeostasis result in profound modulations in protein molecules in a variety of diseases including male infertility. The main focus should be to establish ‘protein signatures’ in infertile patients who exhibit high levels of OS from a lengthy list of proteins identified. This would help not only in pinpointing the exact alteration in underlying molecular mechanism but also benefit clinicians in the diagnosis and prognosis of male factor infertility. In recent years, the advancement in high-throughput proteomic technology has enabled towards gaining a deeper insight into the several protein oxidative modifications known to occur in several diseased states. However, its wider implications in the field of sperm proteomics are still at its infancy. It is hoped that these high-throughput proteomic technologies will help to unravel the underlying molecular mechanism associated with male infertility. In addition, these will also help in understanding if these proteomic biomarkers can be manipulated at the molecular level leading to the establishment of a suitable male contraceptive.
Financial support & sponsorship:
This study was supported by the Department of Science and Technology (INSPIRE Program), Council for Scientific and Industrial Research and University Grants Commission, Government of India, New Delhi.
Conflicts of Interest:
None.
Acknowledgment
Authors thank Dr Ashok Agarwal, Director, American Center for Reproductive Medicine, Cleveland Clinic, Cleveland, Ohio, for his valuable suggestions.
References
- Male contraception: Prospects for sound and ultrasound. Med Hypotheses. 2017;107:1-4.
- [Google Scholar]
- Importance of male fertility control in family planning. Endocr Metab Immune Disord Drug Targets. 2014;14:134-44.
- [Google Scholar]
- Male contraception: A clinically-oriented review. Hormones (Athens). 2015;14:598-614.
- [Google Scholar]
- Is human fecundity changing? A discussion of research and data gaps precluding us from having an answer. Hum Reprod. 2017;32:499-504.
- [Google Scholar]
- An evidence-based perspective on the role of sperm chromatin integrity and sperm DNA fragmentation testing in male infertility. Transl Androl Urol. 2017;6:S665-72.
- [Google Scholar]
- Proteomic analysis of mature and immature ejaculated spermatozoa from fertile men. Asian J Androl. 2016;18:735-46.
- [Google Scholar]
- DNA damage in human spermatozoa; important contributor to mutagenesis in the offspring. Transl Androl Urol. 2017;6:S761-4.
- [Google Scholar]
- Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146-80.
- [Google Scholar]
- Sperm proteome and reproductive technologies in mammals. Anim Reprod Sci. 2016;173:1-7.
- [Google Scholar]
- Proteomics and the genetics of sperm chromatin condensation. Asian J Androl. 2011;13:24-30.
- [Google Scholar]
- Post-translational modifications in sperm proteome: The chemistry of proteome diversifications in the pathophysiology of male factor infertility. Biochim Biophys Acta. 2016;1860:1450-65.
- [Google Scholar]
- Impact of precise modulation of reactive oxygen species levels on spermatozoa proteins in infertile men. Clin Proteomics. 2015;12:4.
- [Google Scholar]
- WHO Laboratory manual for the examination and processing of human semen (5th ed). Geneva: WHO; 2010.
- Clinical utility of sperm DNA fragmentation testing: Practice recommendations based on clinical scenarios. Transl Androl Urol. 2016;5:935-50.
- [Google Scholar]
- Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol. 2016;28:164-71.
- [Google Scholar]
- Male factors in recurrent pregnancy loss. In: Bashiri A, Harlev A, Agarwal A, eds. Recurrent pregnancy loss: Evidence based evaluation, diagnosis and treatment. USA: Springer, Cham; 2016. p. :109-29.
- [Google Scholar]
- Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol Reprod Dev. 2017;84:1039-52.
- [Google Scholar]
- Male infertility: The effectof natural antioxidants and phytocompounds on seminal oxidative stress. Diseases. 2017;5:E9.
- [Google Scholar]
- Oxidative stress, spermatozoa and leukocytic infiltration: Relationships forged by the opposing forces of microbial invasion and the search for perfection. J Reprod Immunol. 2013;100:11-9.
- [Google Scholar]
- Endocrine control of spermatogenesis: Role of FSH and LH/ testosterone. Spermatogenesis. 2014;4:e996025.
- [Google Scholar]
- The unique, complex organization of the transcriptionally silent sperm chromatin. Crit Rev Eukaryot Gene Expr. 1996;6:139-47.
- [Google Scholar]
- Gene transcripts in spermatozoa: Markers of male infertility. Clin Chim Acta. 2012;413:1035-8.
- [Google Scholar]
- Detection of P2 precursors in the sperm cells of infertile patients who have reduced protamine P2 levels. Fertil Steril. 1998;69:755-9.
- [Google Scholar]
- Marked differences in protamine content and P1/P2 ratios in sperm cells from percoll fractions between patients and controls. J Androl. 2003;24:438-47.
- [Google Scholar]
- Protamine 2 precursors (Pre-P2), protamine 1 to protamine 2 ratio (P1/P2), and assisted reproduction outcome. Fertil Steril. 2009;91:715-22.
- [Google Scholar]
- Improvement in chromatin maturity of human spermatozoa selected through density gradient centrifugation. Int J Androl. 2011;34:256-67.
- [Google Scholar]
- Evaluation of sperm proteins in infertile men: A proteomic approach. Fertil Steril. 2011;95:2745-8.
- [Google Scholar]
- Proteomics analysis of good and poor quality human sperm demonstrates several proteins are routinely aberrantly regulated. Biol Reprod. 2017;99:395-408.
- [Google Scholar]
- Comparative analysis of proteomes between diabetic and normal human sperm: Insights into the effects of diabetes on male reproduction based on the regulation of mitochondria-related proteins. Mol Reprod Dev. 2018;85:7-16.
- [Google Scholar]
- Human spermatozoa quantitative proteomic signature classifies normo- and asthenozoospermia. Mol Cell Proteomics. 2017;16:57-72.
- [Google Scholar]
- Differential proteomic profiling of spermatozoal proteins of infertile men with unilateral or bilateral varicocele. Urology. 2015;85:580-8.
- [Google Scholar]
- Identification of several proteins involved in regulation of sperm motility by proteomic analysis. Fertil Steril. 2007;87:436-8.
- [Google Scholar]
- Identification of proteomic differences in asthenozoospermic sperm samples. Hum Reprod. 2008;23:783-91.
- [Google Scholar]
- Proteomics-based study on asthenozoospermia: Differential expression of proteasome alpha complex. Mol Hum Reprod. 2010;16:452-62.
- [Google Scholar]
- Sperm phosphoproteome profiling by ultra performance liquid chromatography followed by data independent analysis (LC-MS(E)) reveals altered proteomic signatures in asthenozoospermia. J Proteomics. 2012;75:5861-71.
- [Google Scholar]
- Major protein alterations in spermatozoa from infertile men with unilateral varicocele. Reprod Biol Endocrinol. 2015;13:8.
- [Google Scholar]
- Proteomic signatures of infertile men with clinical varicocele and their validation studies reveal mitochondrial dysfunction leading to infertility. Asian J Androl. 2016;18:282-91.
- [Google Scholar]
- Proteome analysis of round-headed and normal spermatozoa by 2-D fluorescence difference gel electrophoresis and mass spectrometry. Asian J Androl. 2009;11:683-93.
- [Google Scholar]
- Proteomic profile of seminal plasma in adolescents and adults with treated and untreated varicocele. Asian J Androl. 2016;18:194-201.
- [Google Scholar]
- Proteomic analysis of seminal plasma from normal volunteers and post-vasectomy patients identifies over 2000 proteins and candidate biomarkers of the urogenital system. J Proteome Res. 2011;10:941-53.
- [Google Scholar]
- Development of narrow immobilized pH gradients covering one pH unit for human seminal plasma proteomic analysis. Proteomics. 2003;3:1611-9.
- [Google Scholar]
- Proteomic analysis of seminal plasma in men with different spermatogenic impairment. Andrologia. 2012;44:256-64.
- [Google Scholar]
- Functional proteomic analysis of seminal plasma proteins in men with various semen parameters. Reprod Biol Endocrinol. 2013;11:38.
- [Google Scholar]
- Challenges of proteomic studies in human reproduction. In: Agarwal A, Samanta L, Bertollo PR, Durairaganayagam D, Intasqui P, eds. Proteomics in human reproduction: Biomarkers for millennials. USA: Springer Publications; 2016.
- [Google Scholar]
- Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1-17.
- [Google Scholar]
- Proteomic analysis of human spermatozoa proteins with oxidative stress. Reprod Biol Endocrinol. 2013;11:48.
- [Google Scholar]
- Proteomic analysis of seminal fluid from men exhibiting oxidative stress. Reprod Biol Endocrinol. 2013;11:85.
- [Google Scholar]
- Comparative proteomic network signatures in seminal plasma of infertile men as a function of reactive oxygen species. Clin Proteomics. 2015;12:23.
- [Google Scholar]
- Sperm competition and the evolution of spermatogenesis. Mol Hum Reprod. 2014;20:1169-79.
- [Google Scholar]
- Non-random positioning of chromosomes in human sperm nuclei. Chromosome Res. 2004;12:163-73.
- [Google Scholar]
- Histone retention, protein carbonylation, and lipid peroxidation in spermatozoa: Possible role in recurrent pregnancy loss. Syst Biol Reprod Med. 2016;62:201-12.
- [Google Scholar]
- 3D Chromatin Structures of Mature Gametes and Structural Reprogramming during Mammalian Embryogenesis. Cell. 2017;170:367-81.e20.
- [Google Scholar]
- Epigenetic modifications and reprogramming in paternal pronucleus: sperm, preimplantation embryo, and beyond. Cell Mol Life Sci. 2017;74:1957-67.
- [Google Scholar]
- Establishing Chromatin Regulatory Landscape during Mouse Preimplantation Development. Cell. 2016;165:1375-88.
- [Google Scholar]
- Spermidine promotes mating and fertilization efficiency in model organisms. Cell Cycle. 2013;12:346-52.
- [Google Scholar]






