Translate this page into:
Effect of roscovitine on developmental competence of small follicle-derived buffalo oocytes
For correspondence: Dr Vikash Chandra, Division of Physiology & Climatology, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly 243 122, Uttar Pradesh, India e-mail: vikashvet15@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The lower recovery of competent oocytes in buffalo species limits the commercialization of in vitro embryo production technology in field condition. In this context, pre-maturation of small follicle (SF)-derived oocytes with meiotic inhibition may be a promising alternative to obtain more number of competent oocytes. Thus, the present study was conducted with an objective to enhance the developmental potential of less competent SF-derived buffalo oocytes.
Methods:
All the visible follicles (used for aspiration) from buffalo ovaries were divided into two categories: large follicle (LF) (follicles having diameter ≥6 mm) and SF (follicles of diameter <6 mm). The competence of LF and SF oocytes was observed in terms of brilliant cresyl blue (BCB) staining, cleavage rate, blastocyst rate and relative gene expression of oocyte and blastocyst competence markers. Thereafter, less competent SF oocytes were treated with 0, 12.5, 25, 50 and 100 mM doses of roscovitine (cyclin-dependent kinase inhibitor) to enhance their developmental potential.
Results:
Based on parameters studied, LF oocytes were found to be more competent than SF oocytes. Pre-maturation incubation of SF oocytes with roscovitine reversibly arrested oocyte maturation for 24 h to ensure the proper maturation of less competent oocytes. A significantly higher number of BCB-positive oocytes were noted in roscovitine-treated group than SF group. Cleavage and blastocyst rates were also higher in roscovitine-treated group. The relative messenger RNA expression of oocyte (GDF9, BMP15, GREM1, EGFR, PTGS2 and HAS2) as well as blastocyst (INF-τ, GLUT1 and POU5F1) competence markers was significantly greater in roscovitine-treated group relative to SF group. Again, on comparison with LF group, these parameters depicted a lower value in the treatment group.
Interpretation & conclusions:
The findings of this study has revealed that pre-maturation incubation of SF-derived oocytes with 25 μM roscovitine can improve its developmental competence and thus can be utilized to get maximum number of competent oocytes for better commercialization of in vitro embryo production technology in buffalo.
Keywords
Blastocysts
buffalo
cytoplasmic maturation
developmental competence
oocytes
roscovitine
Substantial research has been conducted for the improvement of culture requirements of buffalo in vitro embryo production (IVEP)1. The major concern is the lower oocytes recovery compared to other species2 which is one of the major constraints in the commercialization of buffalo IVEP technology in field conditions3. The acquisition of developmental competence is a sequential process which occurs along with the follicular growth in ruminants. This developmental process includes both nuclear and cytoplasmic maturation4. Thus, the fully matured oocytes having complete follicular information in the form of messenger RNA (mRNA) or proteins must be collected for improvement in bubaline in vitro culture5. If oocytes are collected before the acquisition of adequate information, developmental potential of embryo decreases6. There are some important events occurring in the oocyte during the late follicular growth, which are essential to achieve full developmental competence7. This has already been proved as cumulus-oocyte complexes (COCs) derived from large follicle (LF, >6 mm) of adult cattle have better cytoplasmic maturation and show higher developmental competence, whereas small follicle (SF, <6 mm)-derived oocytes are less competent due to inadequate cytoplasmic maturation8. Along with bovine species, improved blastocyst rate through selection of competent oocytes from LF has also been reported in bubaline9, caprine10, ovine11, porcine7 and humans12. Hence, blastocyst rate can be improved either by selecting more competent LF oocytes or by ensuring cytoplasmic maturation of SF oocytes.
In most of the mammals, oocytes are maintained at germinal vesicle (GV) stage until pre-ovulatory luteinizing hormone (LH) surge. During this period of meiotic arrest, oocytes undergo morphological and biochemical changes to achieve developmental competence13. However, when oocytes are removed from follicle, they spontaneously resume nuclear maturation with impaired oocyte capacitation and result in lower rate of embryo development13. Competence of SF-derived oocytes may be enhanced by providing sufficient pre-maturation incubation for a specific period of time14 in the presence of meiotic inhibitors such as roscovitine, cycloheximide, 6-dimethylaminopurine and butyrolactone. Amongst all meiotic inhibitors, roscovitine is an effective and reversible inhibitor of cyclin-dependent kinase 215, capable of arresting the cells in late G1 and G2/M cell cycle transition16. It has less detrimental effects on oocyte developmental competence than other inhibitors14 and has been used effectively to reversibly block the nuclear maturation of oocytes for certain time period in bovine141617, equine6, ovine18 and porcine19. Moreover, porcine embryos obtained from oocytes pre-cultured with roscovitine developed to term, making its introduction desirable in assisted reproductive technology programmes20.
These studies reported the reversible inhibitory effect of roscovitine in pooled oocytes derived from the visible ovarian follicles. The present study was carried out to investigate the effect of roscovitine on in vitro maturation of SF-derived buffalo oocytes and further development to the blastocyst stage.
Material & Methods
The study was conducted in the division of Physiology and Climatology, ICAR-Indian Veterinary Research Institute, Izatnagar, Bareilly, India. On the basis of diameter, ovarian follicles were categorized as LF (≥6 mm) and SF (<6 mm)2122. The study was undertaken as three individual experiments: (i) comparative analysis of developmental competence of LF and SF oocytes, (ii) determination of doses of roscovitine to improve the developmental competence of SF-derived oocytes, and (iii) comparison between the developmental correlates of SF, LF and roscovitine-treated SF group.
The best dose of roscovitine was decided based on the following criteria: (i) inhibitory effect of roscovitine (0, 12.5, 25, 50 and 100 μM) treatment for 24 h on nuclear maturation; (ii) reversibility of roscovitine on nuclear maturation after 24 h maturation in roscovitine-free medium; (iii) cleavage rate; and (iv) blastocyst rate. Thereafter, the developmental competence of LF, SF and 25 μM roscovitine-treated COCs was compared on the basis of brilliant cresyl blue (BCB) staining, cleavage rate, blastocyst rate, relative gene expression of oocyte competence markers in cumulus cells and denuded oocytes and blastocyst competence markers.
In vitro embryo production
Oocyte retrieval and grading: Buffalo (Bubalus bubalis) ovaries collected from local abattoir were transported to the laboratory in pre-warmed normal saline solution (NSS) supplemented with 50 μg/ml gentamycin sulphate (Sigma, USA) at 37-39°C, within 2-3 h of slaughter. Ovaries were washed with NSS to remove dirt, blood, extra tissues, etc. The follicular fluid was aspirated separately from LF (≥6 mm) and SF (<6 mm) using 18-gauge needle fitted with 5 ml syringe containing oocyte collection medium [OCM consisting of TCM-199 HEPES modified (Sigma)]. Three mg/ml bovine serum albumin (BSA) (Sigma)2122. All COCs with more than two layers of compact cumulus layers and homogeneous granular ooplasm were used for the study2122.
Treatment of oocytes with roscovitine: COCs derived from SF were rinsed with OCM and then with pre-maturation medium consisting of oocyte collection medium (TCM-199 HEPES) modified, 5 μg/ml luteinizing hormone (LH), 0.5 μg/ml follicle-stimulating hormone (FSH, Sigma), 1 μg/ml estradiol-17β, 20 ng/ml EGF, 0.25 mM sodium pyruvate, 0.68 mM L-glutamine, 10 μg/ml gentamicin sulphate, 3 mg/ml BSA and 10 per cent fetal bovine serum (FBS) and different concentrations of roscovitine (all from Sigma) (0, 12.5, 25, 50 and 100 μM)16. Oocytes were pre-incubated in the 50 μl drop (10-15 COCs each drop) of pre-maturation medium for 24 h at 38.5°C in a 5 per cent CO2, humidified air atmosphere. After 24 h prematuration, one-third of the denuded oocytes (cumulus cells were removed by treating with 0.25% hyaluronidase) were treated with pronase for 30-45 sec and stained with 0.005 per cent ethidium bromide for 10 min at room temperature and washed in phosphate-buffered saline and observed under fluorescent microscope. Nuclear status seen was categorized as the GV, GV breakdown (GVBD), metaphase I (MI), ana-telophase (AT) and metaphase II (MII) (Fig. 1). The remaining two-thirds of the COCs were kept in inhibitor-free medium for additional 24 h period to resume the nuclear maturation.
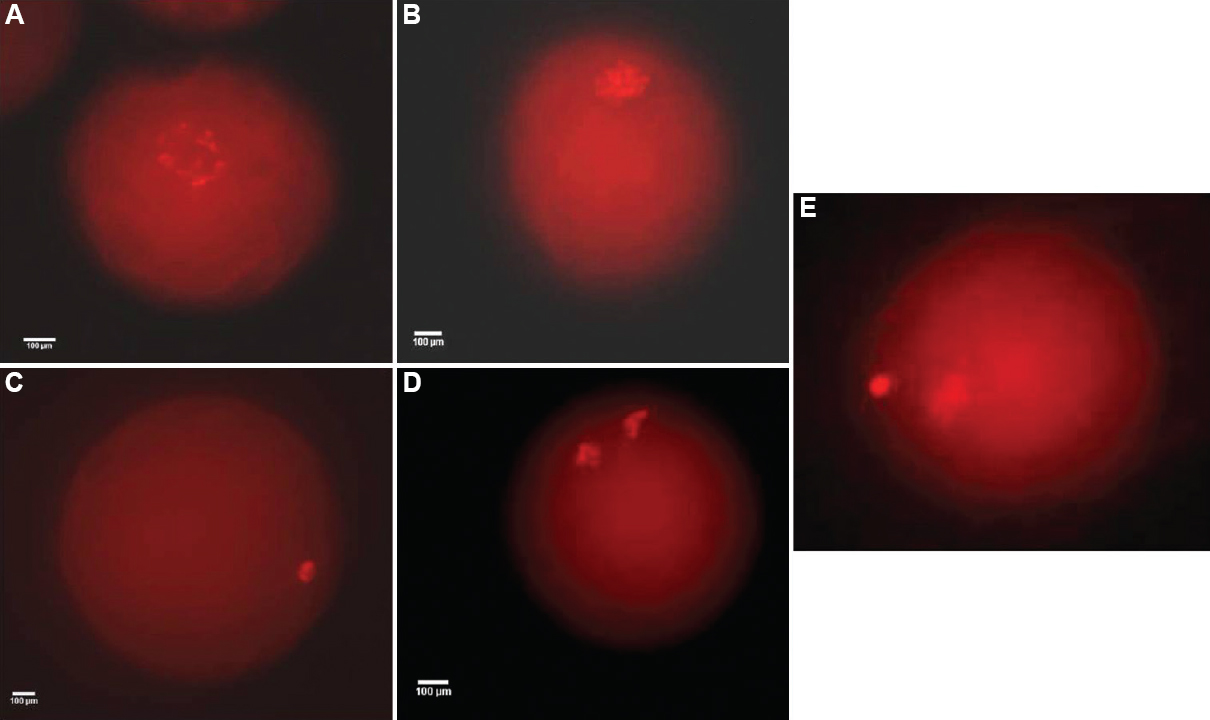
- Different nuclear stages observed after ethidium bromide (EtBr) staining of pre-matured and matured denuded oocyte: (A) germinal vesicle (GV); (B) germinal vesicle breakdown (GVBD); (C) metaphase I (MI); (D) ana-telophase (AT); (E) metaphase II (MII) (×400).
Brilliant cresyl blue (BCB) staining to select competent oocyte: COCs derived from LF (n=97), SF (n=110) and 25 μM roscovitine-treated (n=107) groups were subjected for BCB staining as per the defined protocol2123. The COCs were divided into two groups depending on the colour of cytoplasm; BCB-positive COCs were those with any degree of blue coloration, whereas BCB-negative COCs were without any blue coloration of the cytoplasm (Fig. 2). The percentage of BCB-positive and BCB-negative oocytes was recorded.
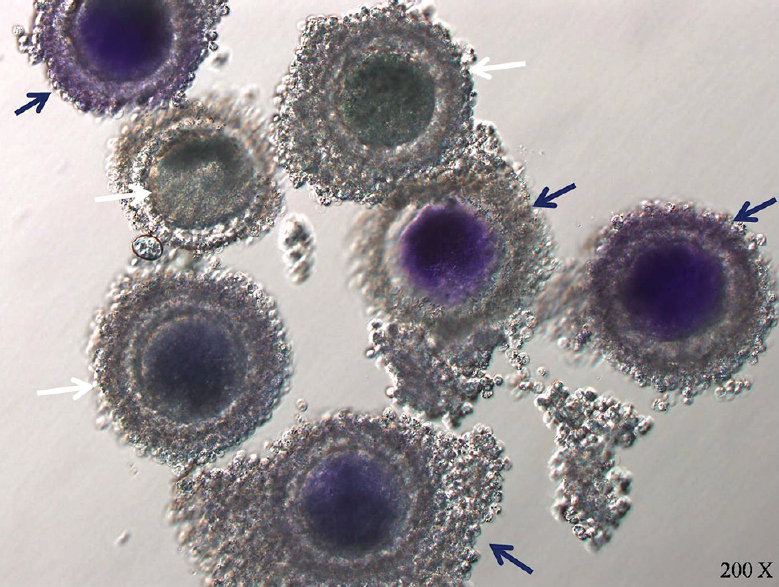
- Brilliant cresyl blue stained oocytes: Blue stained oocytes are designated as brilliant cresyl blue +ve (blue arrow) and colourless oocytes are designated as brilliant cresyl blue −ve (white arrow).
In vitro maturation: After 24 h of roscovitine pre-maturation incubation, COCs were rinsed 4-5 times in maturation medium to avoid the carry-over effect of roscovitine into the final drop of maturation medium. Pre-mature oocytes were subjected to in vitro maturation (IVM) to allow the resumption of meiosis, whereas oocytes derived from LF and control group were directly kept for IVM (no roscovitine treatment) in 50 μl droplets of maturation medium for 24 h at 38.5°C and 5 per cent CO2 in the air with maximum relative humidity.
In vitro fertilization: In vitro fertilization (IVF) was done as per the modified protocol of Pandey et al21. In vitro matured COCs of different groups were subjected toIVF. The frozen buffalo bull semen straws were procured from ICAR-National Dairy Research Institute (ICAR-NDRI), Karnal, India. The semen was washed twice in Tyrode's albumin lactate pyruvate (FerTALP) medium supplemented with 0.2 mM sodium pyruvate, 6 mg/ml BSA and 20 μg/ml heparin (Sigma) and centrifuged at 70 g for 10 min. The pellet formed was suspended in FerTALP, and progressively motile sperm from the supernatant solution was placed as 50 μl droplets having a final concentration of 2×106 spermatozoa/ml. The matured COCs (10-15) were washed, introduced to the semen droplets overlaid with the mineral oil and co-incubated for 18 h with 5 per cent CO2 in air at 38.5°C with maximum relative humidity.
In vitro embryo culture: In vitro embryo culture was done as per the protocol described by Bhardwaj et al23. The presumptive zygotes were washed in modified synthetic oviductal fluid (mSOF) supplemented with 3 mg/ml BSA (fatty acid free), 0.25 mM sodium pyruvate with one per cent (v/v) essential (Sigma) and non-essential amino acids (Sigma), 100 ng/ml IGF1 (Sigma), 0.68 mM L-glutamine and 50 μg/ml gentamycin sulphate. The presumptive zygotes (10-15) were transferred in 50 μl drops of mSOF without FBS for an initial development of 48 h and further cultured in mSOF supplemented with 10 per cent FBS. Medium was replaced at alternate day till blastocyst development or day 8 post-IVF. Group of healthy blastocysts has been depicted in Fig. 3.
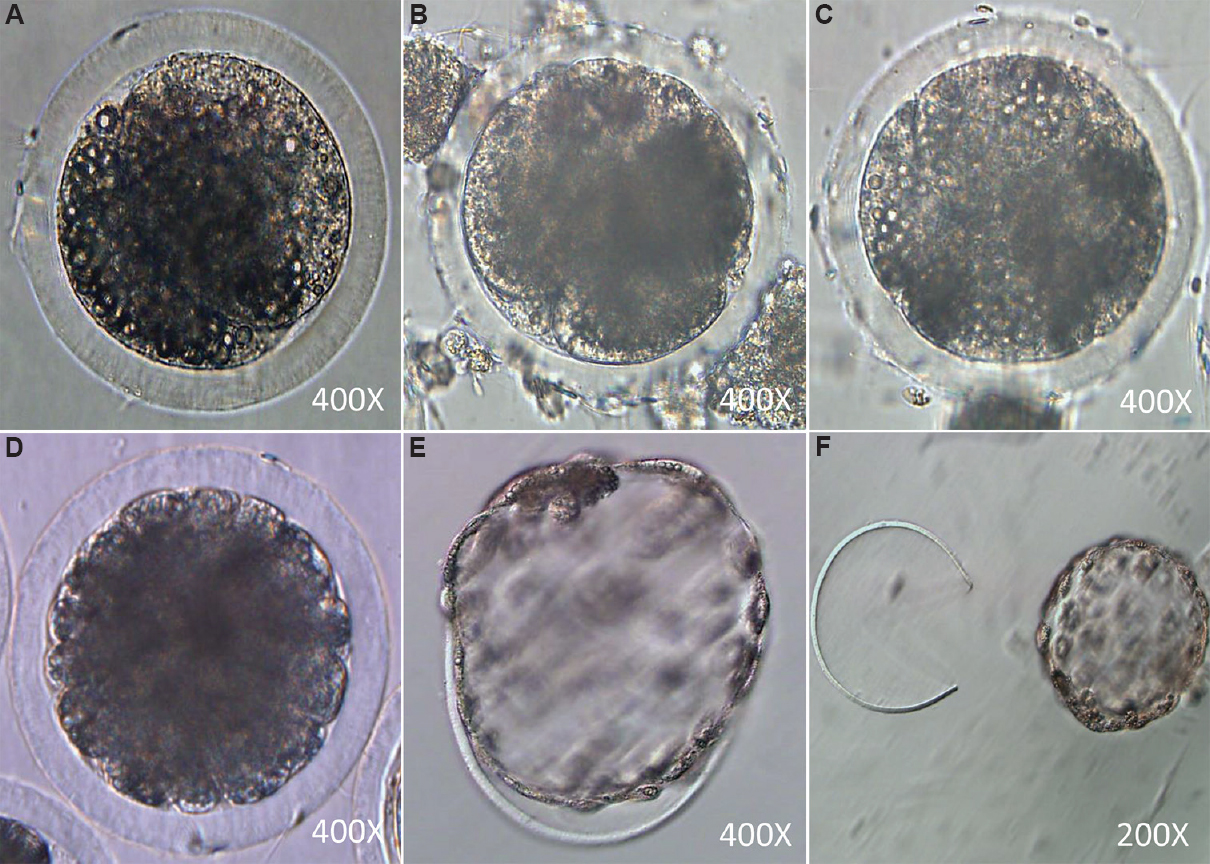
- Different embryonic developmental stages: (A) two-cell stage; (B) four-cell stage; (C) eight-cell stage; (D) Morula stage; (E) hatching blastocyst; (F) hatched blastocyst.
Relative gene expression of oocyte and blastocyst competence markers: The gene-specific primers (RPS15A, HAS2, EGFR, GREM1, PTGS2, GDF9, BMP15, INF-τ, GLUT1 and POU5F1) along with specific annealing temperature and product length were obtained from published sources2124.
Total RNA extraction and cDNA synthesis: The total RNA was harvested in triplicate from denuded oocytes (n=100), cumulus cells isolated from COCs (n=100) and blastocysts (n=10) of respective groups using Trizol reagent (Ambion, USA). To assess the quality and integrity of the RNA, 5 μl of total RNA was subjected to denaturation in one per cent agarose gel electrophoresis. The purity and concentration of total RNA were checked using the NanoDrop Spectrophotometer (Thermo Scientific, USA) and samples with OD260: OD280 values between 1.8 and 2.0 were used for cDNA synthesis. cDNA synthesis was done using Verso cDNA synthesis kit (AB-1453/B; Thermo Scientific, California, USA) with total 20 μl reaction volume following the manufacturer's protocol. A total of 0.2 μg total RNA was used for reverse transcription as a template. The RNA was subsequently reverse transcribed by incubating at 42°C for 59 min followed by the final termination of the reaction by heating for two minutes at 95°C. The cDNA was properly labelled and stored at −20°C for later use. RPS15A was used as housekeeping gene.
Polymerase chain reaction (PCR) amplification: Polymerase chain reaction (PCR) amplification was done in Bioer XP cycler PCR machine (Hangzhou Bloer Technology Co., Ltd., Zhejiang, China) with the amplification reaction mixture of 20 μl, consisting of the 18 μl Platinum PCR mix (Life Technologies-11306-016, California, USA), 0.5 μl each forward and reverse primer of 0.5 μM concentration and 1 μl cDNA. PCR reaction was performed as the initial denaturation step at 94°C for two minutes followed by cDNA amplification cycles, including denaturation at 94°C for 30 sec, annealing at a specific annealing temperature of a primer for 30 sec and extension at 72°C for one minute. No template control (NTC) was maintained for each set of primers, and PCR products were analyzed by electrophoresis on 1.8 per cent agarose gel with LabSafe nucleic acid stain (G-Biosciences) and 50 bp ladder (Fermentas).
Real-time quantitative polymerase chain reaction (qPCR): Quantitative real-time PCR (qPCR) was performed with SYBR green master mix qPCR kit (Thermo Scientific, USA) and SmartCycler Real-Time qPCR (Cepheid, USA). NTC was placed with each reaction setup for checking any contamination in reaction components. Master mix total reaction volume of 20 μl was prepared by adding 10 μl 2X SYBR green mix, 0.5 μl each forward and reverse primer (0.5 μM each), 1 μl cDNA template and 8 μl NFW. RPS15A was taken as the housekeeping gene and cycle threshold (Ct) values, amplification plot and dissociation curve for all the required transcripts were acquired. The Pfaffl25 method of relative quantification was used for calculation of relative gene expression.
Statistical analysis: The data for nuclear status were analyzed by Chi-square test using SAS 9.2 software (SAS Institute Inc., Cary, NC, USA) at 5 per cent level of significance. Data for cleavage rate and blastocyst rate were analyzed by one-way ANOVA with Duncan post hoc test. The change in relative expression of different genes in relation to RPS15A was analyzed by one-way ANOVA with Duncan post hoc test using GraphPad Prism V 5.0 software (GraphPad Software, CA, USA).
Results
The present study was conducted to analyze the effect of roscovitine on the developmental potential of SF-derived less competent oocytes, as during the initial experiments, the higher competence of LF oocytes was noted in comparison to SF oocytes.
Comparison of developmental competence between large follicle and small follicle (LF and SF): LF-derived oocytes were found to be developmentally more competent in terms of higher BCB-positive oocyte (LF-75.2% and SF-29.09%), cleavage rate and blastocyst rate than the SF-derived oocytes. The relative mRNA study also revealed higher developmental potential of LF oocytes than SF oocytes in terms of upregulated expression of the competence markers such as GDF9 and BMP15 in oocyte; GREM1, PTGS2, HAS2 and EGFR in cumulus cells and interferon-t, GLUT1 and POU5F1 (Figs. 4–6).
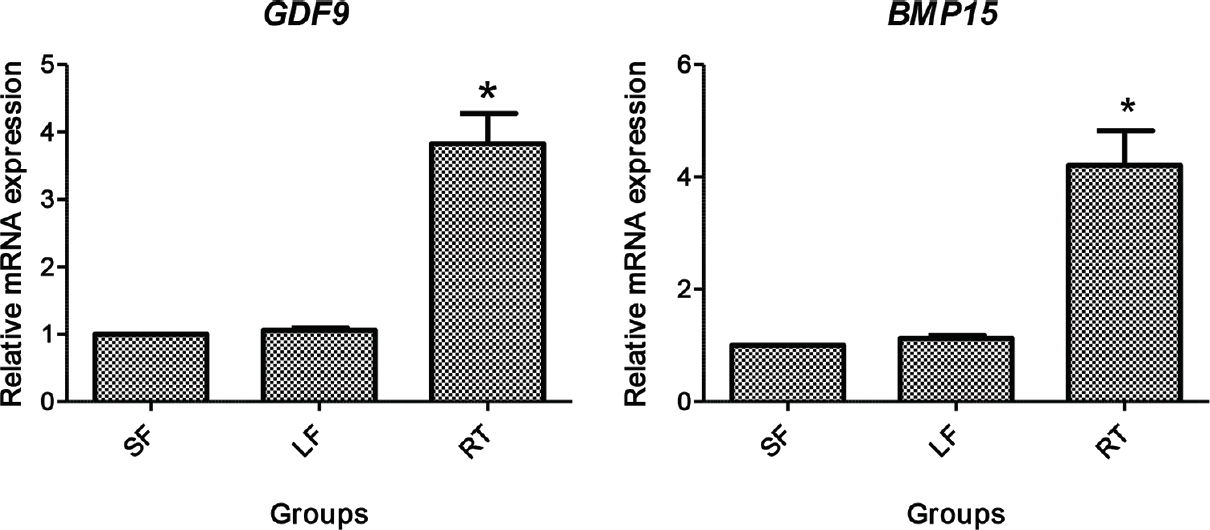
- Relative gene expression of GDF9 and BMP15 in denuded oocytes. SF, small follicle derived; LF, large follicle derived; RT, roscovitine treated. *P<0.05 compared to SF and LF.
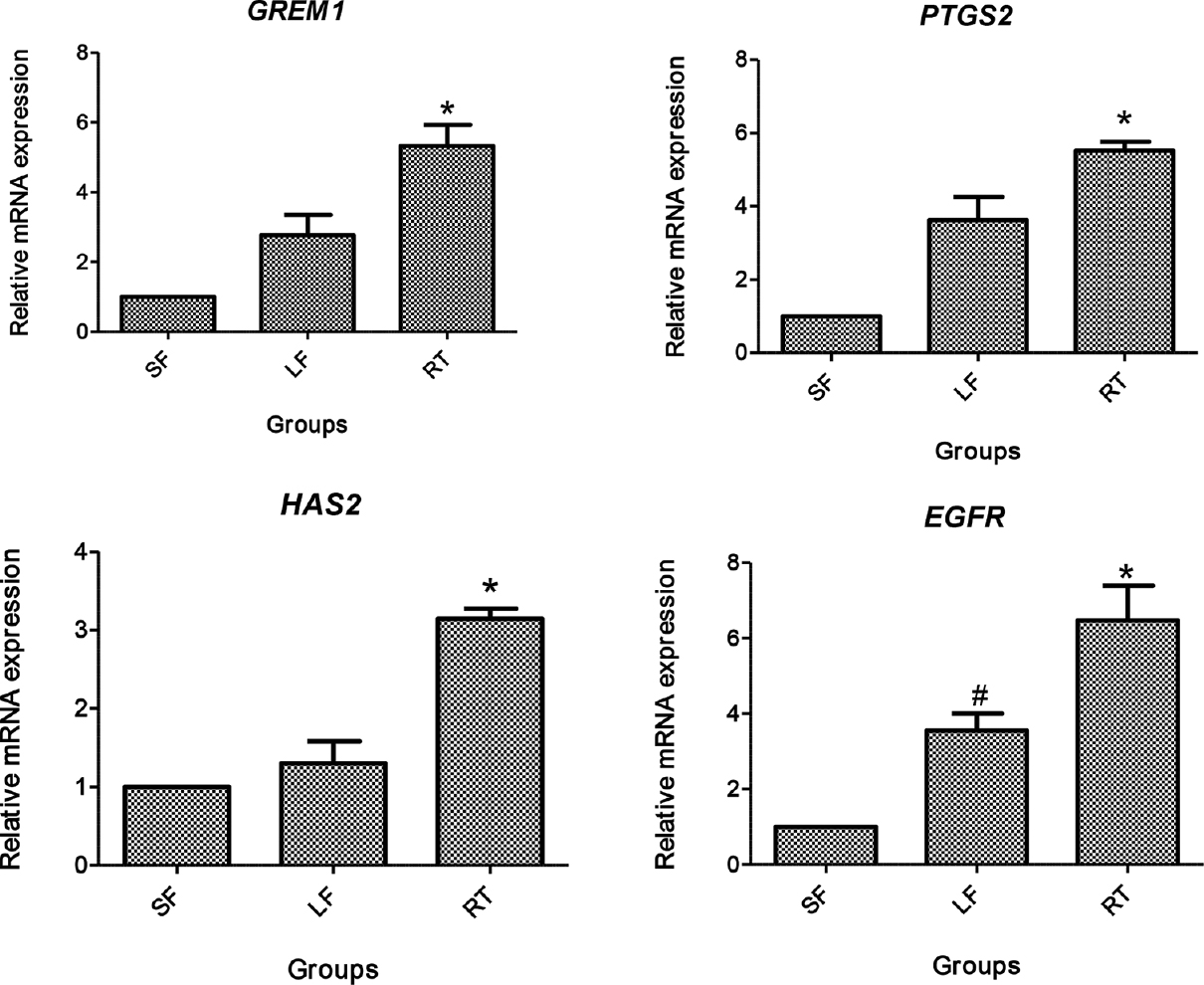
- Relative gene expression of GREM1, PTGS2, HAS2 and epidermal growth factor receptor (EGFR) in cumulus cells. SF, small follicle derived; LF, large follicle derived; RT, roscovitine treated. *P<0.05 compared to SF and LF; #P<0.05 compared to SF.
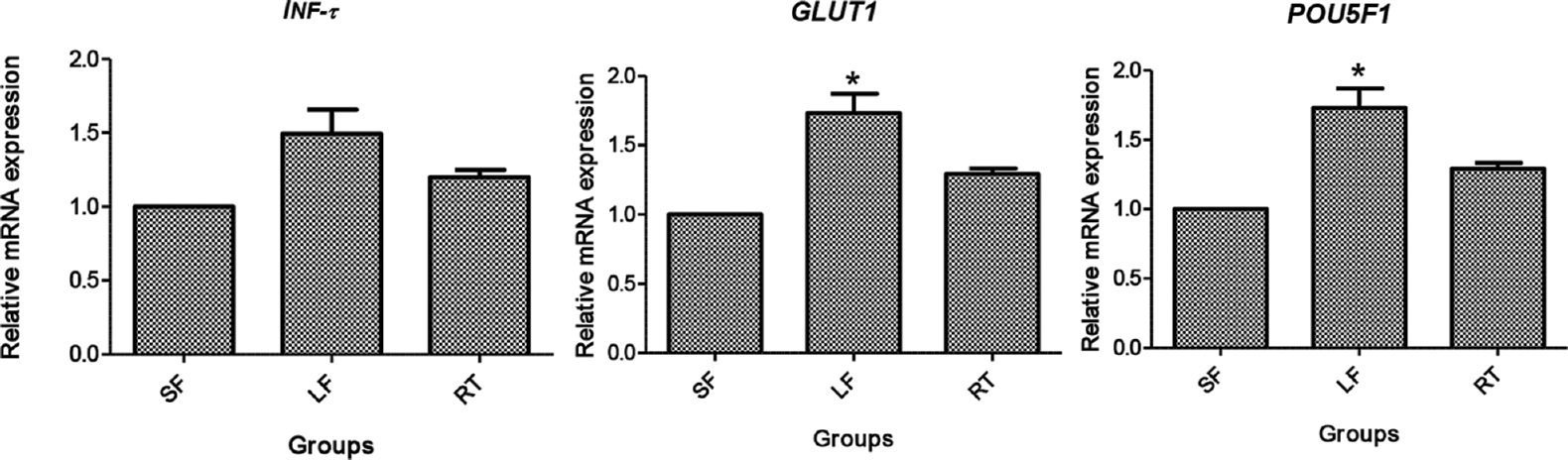
- Relative gene expression of INF-τ, GLUT1 and POU5F1 in blastocysts. SF, small follicle derived; LF, large follicle derived; RT, roscovitine treated. *P<0.05 compared to SF and RT.
Selection of best dose of roscovitine: Different concentrations of roscovitine were tested to select the best dose.First, the inhibition of cumulus expansion after pre-maturation incubation was determined. Roscovitine effectively arrested the cumulus cells’ expansion in a dose-dependent manner. The number of COCs showing cumulus expansion was apparently lowest in 100 μM followed by 50 μM and 25 μM groups than 12.5 μM group. Thus, the maximum inhibition of cumulus expansion was observed in 100 μM roscovitine group, followed by 50 and 25 μM, and the least inhibitory effect was noted in 12.5 μM group (Fig. 7).
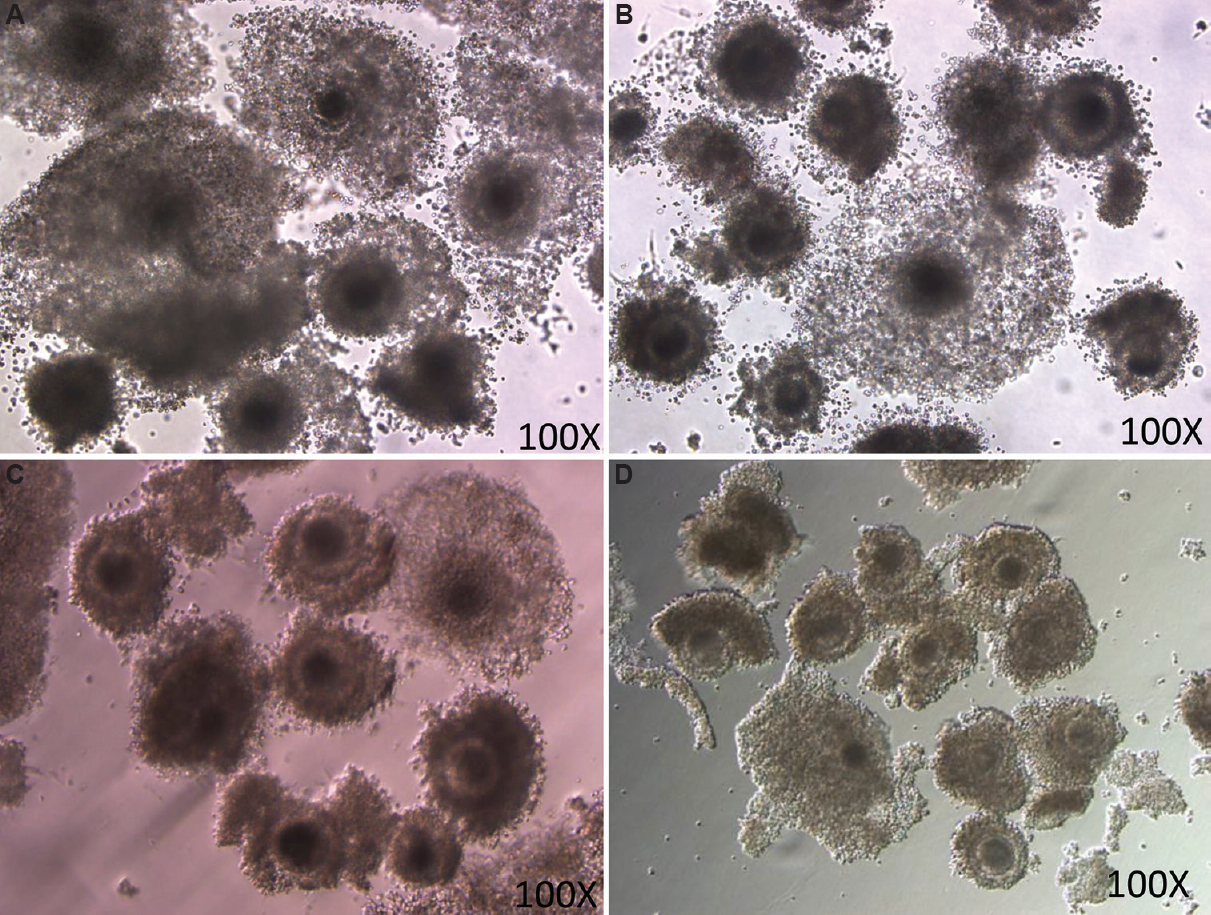
- Cumulus expansion in different groups. COCs treated with different doses of roscovitine- (A) 12.5 μM; (B) 25 μM; (C) 50 μM; and (D) 100 μM.
Second, data of the dose-dependent inhibitory effect of roscovitine were observed. Concentrations of 25, 50 and 100 μM roscovitine exhibited higher percentage of oocytes at GV and GVBD stages than control and 12.5 μM roscovitine (Table I). A comparatively smaller proportion of oocytes reached MII stage with these concentrations of roscovitine. Third, the reversible effect of roscovitine in the oocytes was observed through nuclear staining after 24 h of inhibitor-free maturation. All the groups depicted non-significant difference amongst themselves, but the concentration of 100 μM roscovitine revealed significantly (P<0.05) less number of MII oocytes in comparison to other groups (Table II). Finally, the cleavage and blastocyst rates of different groups were analyzed, and data are provided in Table III. The data depicted significantly (P<0.05) higher cleavage rate in 25 and 50 μM roscovitine-treated groups; however, no significant difference observed amongst control and 12.5 and 100 μM roscovitine-treated groups. A significantly (P<0.05) higher blastocyst rate was observed in 25 and 50 μM roscovitine-treated groups than control and 12.5 μM roscovitine-treated groups which reduced significantly (P<0.05) in 100 μM roscovitine-treated group though it was significantly (P<0.05) higher than the control and 12.5 μM roscovitine-treated group (Table III).
| ROS (μM) | COCs, n# | GV, n (%) | GVBD, n (%) | MI, n (%) | AT, n (%) | MII, n (%) | Unstained, n (%) |
|---|---|---|---|---|---|---|---|
| 0 | 97 | 1 (1.03)a | 2 (2.06)a | 5 (5.15)a | 7 (7.22)a | 72 (74.23)a | 10 (10.31)a |
| 12.5 | 85 | 20 (23.53)b | 4 (4.7)b | 19 (22.35)b | 15 (17.65)b | 20 (23.53)b | 7 (8.24)a |
| 25 | 89 | 67 (75.28)c | 8 (8.99)c | 3 (3.37)a | 3 (3.37)a | 2 (2.25)c | 6 (6.74)a |
| 50 | 97 | 77 (79.38)c | 9 (9.28)c | 2 (2.06)a | 1 (1.03)a | 1 (1.03)c | 7 (7.22)a |
| 100 | 78 | 65 (83.34)c | 4 (5.13)b,c | 1 (1.28)a | 1 (1.28)a | 1 (1.28)c | 6 (7.69)a |
Values (mean%) in the same column with different superscripts differ significantly (P<0.05). #COCs obtained from 5 replicates for each group performed on different days. ROS, roscovitine; COCs, cumulus-oocyte complexes; GV, germinal vesicle; GVBD, germinal vesicle breakdown; MI, meiosis I; AT, ana-telophase; MII, meiosis II
| ROS (μM) | COCs n# | GV, n (%) | GVBD, n (%) | MI, n (%) | AT, n (%) | MII, n (%) | Unstained, n (%) |
|---|---|---|---|---|---|---|---|
| 0 | 91 | 1 (1.1)a | 3 (3.3)a | 7 (7.69)a | 10 (10.99)a | 64 (70.33)a | 6 (6.59)a |
| 12.5 | 82 | 0 (0.0)a | 4 (4.88)a | 9 (10.98)a,b | 10 (12.2)a | 50 (60.98)a | 9 (10.98)a |
| 25 | 92 | 2 (2.17)a | 2 (2.17)a | 12 (13.04)a,b | 14 (15.22)a,b | 55 (59.78)a | 7 (7.61)a |
| 50 | 81 | 0 (0.0)a | 3 (3.70)a | 10 (12.35)a,b | 11 (13.58)a,b | 50 (61.72)a | 7 (8.64)a |
| 100 | 85 | 3 (3.53)a | 7 (8.24)a | 15 (17.65)b | 4 (4.71)b | 37 (43.53)b | 19 (22.35)b |
Values (mean%) in the column with different superscripts differ significantly (P<0.05)
#COCs obtained from 5 replicates for each group performed on different days.
Abbreviations as given in Table I
| Group | COCs, n# | Cleavage rate*, n (%) | Blastocyst rate$, n (%) |
|---|---|---|---|
| SF/0 μM RT | 410 | 152 (37.07±1.4) | 22 (14.47±0.9)a |
| 12.5 μM RT | 190 | 61 (38.27±1.4)a | 19 (14.96±1.7)a |
| 25 μM RT | 228 | 108 (47.56±1.5) | 31 (29.02±1.0)b |
| 50 μM RT | 274 | 136 (51.02±1.7)b | 41 (30.56±1.4)b |
| 100 μM RT | 147 | 58 (40.83±2.2)a | 9 (19.27±1.0)c |
| LF | 248 | 153 (61.69±0.8)c | 56 (36.6±1.4)d |
*,$Values (mean±SE%) in the column with different superscripts differ significantly (P<0.05). #COCs obtained from five replicates for each group performed on different days; *On the basis of total COCs cultured; $On the basis of total oocytes cleaved. RT; roscovitine treated; COCs, cumulus-oocyte complexes; LF, large follicle; SF, small follicles
On the basis of the above findings, 25 μM roscovitine was selected as the minimum effective dose for the improvement of developmental competence of oocytes derived from the SF.
Comparison of best-selected dose of roscovitine with large follicle (LF)-derived oocytes: COCs pre-treated with 25 μM roscovitine were compared with LF-derived COCs in terms of the BCB staining, cleavage rate, blastocyst rate and relative mRNA expression of oocyte and blastocyst competence markers. BCB staining revealed that mean per cent of BCB-positive oocytes was significantly (P<0.05) higher in LF (75.26%) and 25 μM roscovitine-treated (64.49%) groups than SF (29.09%) group. However, no difference was noted between LF and treatment groups. The cleavage and blastocyst rate was significantly higher (P<0.05) in 25 μM roscovitine-treated group than the SF group but was significantly lower (P<0.05) than the LF group (data not shown).
PCR reaction and agarose gel electrophoresis confirmed predicted amplification of primers of respective genes. The relative mRNA expression of oocyte competence markers in denuded oocytes (GDF9 and BMP15) and cumulus cells (GREM1, EGFR, PTGS2 and HAS2 are given in Figs. 4 and 5, respectively. The mRNA expression of GDF9 and BMP15 transcript was significantly higher (P<0.05) in denuded oocytes of roscovitine-treated group than LF and SF groups, whereas no significant difference was observed in GDF9 and BMP15 expression between the LF and SF groups (Fig. 4). The mRNA expression level of GREM1, PTGS2, HAS2 and EGFR in cumulus cells was significantly higher (P<0.05) in roscovitine-treated group than LF and SF groups. Similarly, a significantly higher (P<0.05) expression of GREM1, PTGS2 and EGFR was recorded in LF group than SF group, whereas no difference was noticed in the expression of HAS2 transcript (Fig. 5).
The relative mRNA expression of blastocyst competence markers has been provided in Fig. 6. GLUT1 and POU5F1 revealed a significantly higher (P<0.05) expression in LF group, followed by roscovitine-treated group than the SF group, whereas INF-τ expression was insignificant amongst the groups.
Discussion
Many studies61416171819 have already been conducted to enhance the developmental competence of oocytes by the use of various nuclear inhibitors including roscovitine, but none conducted specifically to enhance the developmental competence of SF-derived oocytes. Hence, the present study was undertaken to enhance the developmental competence of SF-derived oocytes by pre-maturation incubation with roscovitine. During the study, the effect of the different concentrations of roscovitine on developmental competence of SF-derived buffalo oocytes was evaluated in terms of reversible nuclear maturation inhibition, embryo development and relative gene expression of oocyte and blastocyst competence markers, with its further comparison with LF-derived oocytes.
Cumulus expansion was better after 24 h of in vitro maturation in LF oocytes than SF oocytes; also, more number of BCB-positive oocytes was obtained from LF in comparison to the SF group. A similar result has been reported in ovine species26. BCB staining has already been employed as a non-invasive, indirect method for selection of more competent oocytes in various species12728. The present study also reported a significantly higher cleavage and blastocyst rate in LF oocytes than those of SF-derived oocytes. A relationship between follicle size and oocyte quality has been demonstrated in several species. This is due to the greater storage of the transcripts and proteins in the cytoplasm of LF oocytes29.
Results of roscovitine treatment revealed a dose-dependent inhibition of meiotic resumption, and this meiotic inhibition was reversible for all the doses of roscovitine. However, a concentration of 25 μM was found to be the minimum effective dose at which about 75 per cent of the oocytes were arrested at GV stage. The results of the present study were supported by previous studies1417 which reported that 25 μM roscovitine maintained the cattle oocytes at GV stage for 24 h. Albarracin et al16 found 50 μM roscovitine as the best dose for calf oocytes. There are reports in equine6 where oocyte nuclear maturation was inhibited with 50 μM roscovitine, whereas in swine, 80 μM roscovitine30 and 50 μM roscovitine19 were the best doses to inhibit the meiotic resumption. Further, Zhang et al31 concluded that 25 μM roscovitine improved the developmental competence of porcine oocyte. In ovine, 75 μM roscovitine was found to be efficient to reversibly block the meiotic resumption without any detrimental effect on development and quality of in vitro-produced embryos18.
BCB staining and embryo development rate was analyzed in roscovitine-treated group. The dose of 25 μM depicted better BCB staining and embryo development and thus selected as best roscovitine dose because it was the minimum dose which effectively arrested the oocyte meiotic division and significantly improved the in vitro developmental potential of SF-derived oocytes as well as embryos. It has been demonstrated that bovine oocytes arrested with roscovitine and butyrolactone I (BL-I) are competent to undergo normal early development and embryo derived from these oocytes developed into normal foetuses up to day 2732. Kaedi et al33 found an enhanced blastocyst formation rate of reconstructed embryos derived from oocytes pre-cultured with roscovitine. In the present study, roscovitine pretreatment was given only to the SF-derived oocytes, which had a compromised developmental competence, and thus, during the pre-maturation incubation, these might have attained better cytoplasmic maturation and in turn revealed greater competence than untreated SF oocytes.
The enhanced relative expression of competence markers such as GDF9 and BMP15 in oocyte and GREM1, PTGS2 and EGFR in cumulus cells depicted the role of 25 μM roscovitine in enhancing oocyte competence. Lequarre et al34 also reported the increased mRNA expression in roscovitine-treated bovine oocytes. This may define the process of neotranscription, or the addition of poly(A) tails to transcripts not yet adenylated, or both in the GV-arrested oocytes. This effect of roscovitine is transient as it is no longer detectable after 24 h of maturation31. Hence, the significantly upregulated expression of the studied transcripts in roscovitine-treated group may be related to the neotranscription or increased polyadenylation.
Further, the blastocyst derived from LF and roscovitine-treated SF group showed significantly higher expression of blastocyst competence markers (GLUT1 and POU5F1) than SF group, which suggested that roscovitine treatment enhanced the developmental potential of SF-derived oocytes in terms of blastocysts number as well as its quality.
Hence, it is concluded from the study that 25 μM roscovitine effectively improved the developmental potential of less competent SF oocytes as it enhanced the mRNA expression pattern of competence-related molecules. It provides a useful method for ensuring the availability of more number of competent oocytes for buffalo embryo transfer technology as well as an efficient tool for transportation or manipulation of oocytes at the onset of maturation.
Financial support & sponsorship:
The study was supported by funding received from the ICAR-IVRI, Izatnagar under project (grant no. IVRI/P&C/13-15/005).
Conflicts of Interest:
None.
Acknowledgment
The authors acknowledge the Director, ICAR-Indian Veterinary Research Institute (ICAR-IVRI), Izatnagar, for providing necessary facilities for conducting this work.
References
- Stem cell conditioned media contains important growth factors and improves in vitro buffalo embryo production. Anim Biotechnol. 2016;27:118-25.
- [Google Scholar]
- In vitro embryo production in buffalo species: State of the art. Theriogenology. 2002;57:237-56.
- [Google Scholar]
- In vitro embryo production in buffalo: Yesterday, today and tomorrow. Buff Bull. 2013;32(Special Issue I):188-95.
- [Google Scholar]
- The use of genomics and proteomics to understand oocyte and early embryo functions in farm animals. Reprod Suppl. 2003;61:117-29.
- [Google Scholar]
- Effects of roscovitine on maintenance of the germinal vesicle in horse oocytes, subsequent nuclear maturation, and cleavage rates after intracytoplasmic sperm injection. Reproduction. 2003;125:693-700.
- [Google Scholar]
- Effect of follicular size on meiotic and developmental competence of porcine oocytes. Theriogenology. 2002;57:1523-32.
- [Google Scholar]
- Influence of antral follicle size on oocyte characteristics and embryo development in the bovine. Theriogenology. 2005;63:841-59.
- [Google Scholar]
- Follicle size and oocyte diameter in relation to developmental competence of buffalo oocytes in vitro . Reprod Fertil Dev. 2002;14:55-61.
- [Google Scholar]
- Effect of follicle size on recovery and in vitro maturation of black Iraqi goat oocytes. Al Anbar J Vet Sci. 2012;5:130-3.
- [Google Scholar]
- Effect of follicular size on in vitro maturation, fertilization and culture of sheep embryos. Iran J Vet Res. 2013;14:299-304.
- [Google Scholar]
- The developmental competence of oocytes retrieved from the leading follicle in controlled ovarian stimulated cycles. Int J Fertil Steril. 2013;6:272-7.
- [Google Scholar]
- Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol Reprod Dev. 2002;61:234-48.
- [Google Scholar]
- High developmental competence of cattle oocytes maintained at the germinal vesicle stage for 24 hours in culture by specific inhibition of MPF kinase activity. Mol Reprod Dev. 2000;55:89-95.
- [Google Scholar]
- The specificities of protein kinase inhibitors: An update. Biochem J. 2003;371:199-204.
- [Google Scholar]
- Effects of ROS on the nuclear and cytoskeletal components of calf oocytes and their subsequent development. Theriogenology. 2005;64:1740-55.
- [Google Scholar]
- Role of roscovitine and IBMX on kinetics of nuclear and cytoplasmic maturation of bovine oocytes in vitro . Anim Reprod Sci. 2007;99:202-7.
- [Google Scholar]
- In vitro developmental competence of adult sheep oocytes treated with roscovitine. Reprod Domest Anim. 2016;51:276-81.
- [Google Scholar]
- In vitro maturation and fertilization of porcine oocytes after a 48 h culture in roscovitine, an inhibitor of p34cdc2/cyclin B kinase. Anim Reprod Sci. 2006;92:321-33.
- [Google Scholar]
- Birth of piglets after transferring of in vitro-produced embryos pre-matured with R-roscovitine. Reproduction. 2005;129:747-55.
- [Google Scholar]
- Comparative analysis of developmental and molecular correlates of developmental competence of buffalo oocytes derived from small and large follicles. Indian J Anim Sci. 2017;87:1194-9.
- [Google Scholar]
- Impact of oocyte-secreted factors on its developmental competence in buffalo. Zygote. 2017;25:313-20.
- [Google Scholar]
- GREM1, EGFR, and HAS2; the oocyte competence markers for improved buffalo embryo production in vitro . Theriogenology. 2016;86:2004-11.
- [Google Scholar]
- Isolation and characterization of buffalo Wharton's jelly derived mesenchymal stem cells. J Stem Cell Res Ther. 2014;4:207.
- [Google Scholar]
- A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45.
- [Google Scholar]
- In vitro developmental competence of bovine oocytes: Effect of corpus luteum and follicle size. Iran J Reprod Med. 2015;13:615-22.
- [Google Scholar]
- Brilliant cresyl blue stain selects largest oocytes with highest mitochondrial activity, maturation-promoting factor activity and embryo developmental competence in prepubertal sheep. Reproduction. 2011;142:517-27.
- [Google Scholar]
- Lipid content, active mitochondria and brilliant cresyl blue staining in bovine oocytes. Theriogenology. 2013;79:417-22.
- [Google Scholar]
- Genome activation and developmental block in bovine embryos. Anim Reprod Sci. 2004;82-83:13-20.
- [Google Scholar]
- Alterations and reversibility in the chromatin, cytoskeleton and development of pig oocytes treated with roscovitine. Mol Reprod Dev. 2003;64:482-91.
- [Google Scholar]
- Meiotic arrest with roscovitine and follicular fluid improves cytoplasmic maturation of porcine oocytes by promoting chromatin de-condensation and gene transcription. Sci Rep. 2017;7:11574.
- [Google Scholar]
- Embryonic and foetal development of bovine oocytes treated with a combination of butyrolactone I and roscovitine in an enriched medium prior to IVM and IVF. Mol Reprod Dev. 2002;62:513-8.
- [Google Scholar]
- Effect of roscovitine pretreatment on the meiotic maturation of bovine oocytes and their subsequent development after somatic cell nuclear transfer. J Anim Vet Adv. 2010;9:2848-53.
- [Google Scholar]
- Poly(A) RNA is reduced by half during bovine oocyte maturation but increases when meiotic arrest is maintained with CDK inhibitors. Biol Reprod. 2004;71:425-31.
- [Google Scholar]






