Translate this page into:
Do the clonally different Escherichia coli isolates causing different infections in a HIV positive patient affect the selection of antibiotics for their treatment?
*For correspondence: n_arunagiri@yahoo.co.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
In HIV patients, bacterial infections are mostly caused by the pathogenic bacteria harbouring multidrug-resistance genes1. While carrying out a study (2014-2015) on infections caused by drug-resistant bacteria in HIV positive patients attending YRG Centre for AIDS Research and Education (YRG CARE), Chennai, Tamil Nadu, India, we found a 35 yr old male patient who had complaints of severe fever, irritation during micturition, vomiting and body chills. Hence, the urine and blood specimens were collected from this patient, and were subjected to bacterial culture and identification of the isolates. This patient was also infected with leptospirosis, Pneumocystis jirovecii pneumonia and pulmonary tuberculosis. He was under antiretroviral therapy with the following regimen: tenofovir, emtricitabine and efavirenz and was hospitalized for nine weeks. The CD4 cell count of this patient at the time of admission was 19 cells/μl. The bacterial isolates from both the specimens were identified as Escherichia coli based on the standard cultural and biochemical characteristics. Both these E. coli isolates were subjected to polymerase chain reaction-random amplified polymorphic DNA (PCR-RAPD) analysis to determine their clonal relationship and molecular detection of drug-resistance genes using PCR and DNA gene sequencing. The PCR-RAPD reaction was performed using a 10 base pair primer with the sequence of 5'-AGC GTC ACT G-3' (Eurofins, India)2. Antibiotic susceptibility patterns of these E. coli isolates were studied using the Kirby-Bauer disc diffusion method according to Clinical and Laboratory Standards Institute (CLSI) guidelines3. The genes responsible for the production of extended spectrum β-lactamases (ESBL), such as blaTEM4, blaCTX-M5, blaSHV4 and blaOXA4, metallo β-lactamases6, AmpC β-lactamases7, Class 1 and Class 2 integrons8 and sulphamethoxazole-trimethoprim (TMP-SMX)91011, were detected using PCR technique. Both the E. coli isolates showed different clonal patterns which indicated that the patient had blood and urinary tract infections caused by two different E. coli strains (Figure). The molecular characterization revealed that the E. coli isolate from urine sample harboured the genes blaTEM, blaCTX-M and blaOXA for ESBL and sul1 and sul2 genes for SMX and none for AmpC and Class 1 and Class 2 integrons. The phenotypic characterization showed that the isolate had resistance to ampicillin, doxycycline, gentamicin, cefpodoxime, cefoperazone, ceftriaxone, ciprofloxacin, trimethoprim-sulphamethoxazole, piperacillin, piperacillin-tazobactam, tetracyclin, trimethoprim, cefotaxime, ceftazidime and cefoxitin and sensitivity to amikacin, chloramphenicol, ertapenem and imipenem. On the other hand, the E. coli isolate from blood sample was found to harbour the genes blaCTX-M for ESBL, blaCIT-M for AmpC, sul1, sul2 and dfrA7 for TMP-SMX and also for Class 2 integron by molecular characterization and also showed resistance to ampicillin, aztreonam, cefpodoxime, cefoperazone, ceftriaxone, imipenem, piperacillin, piperacillin-tazobactam, trimethoprim, trimethoprim-sulphamethoxazole, cefotaxime, ceftazidime and cefoxitin and sensitivity to amikacin, doxycycline, chloramphenicol, ciprofloxacin, ertapenem, gentamicin and tetracycline by phenotypic characterization (Table). Both the isolates were negative for MBL-producing genes blaIMP, blaVIM, blaSIM, blaSPM, blaGIM and blaNDM, TMP resistance genes dfrA1, dfrA5 and dfrA17 and for Class 1 integron gene. In our study, β-lactamases-producing genes from both the E. coli isolates were sequenced and identified as blaTEM-116, blaCTX-M-15, blaOXA-1 and blaCMY-30 using BLAST and phylogenetic analyses12. Similar to our study, the co-positivity of ESBL along with AmpC and other drug resistance genes in bacterial isolates from HIV patients was observed by Padmavathy et al13.

-
Escherichia coli isolates from urine and blood samples of a HIV patient showing different gene patterns by polymerase chain reaction-random amplified polymorphic DNA analysis. M, marker; 138 - E. coli isolate from urine; 139 - E. coli isolate from blood.
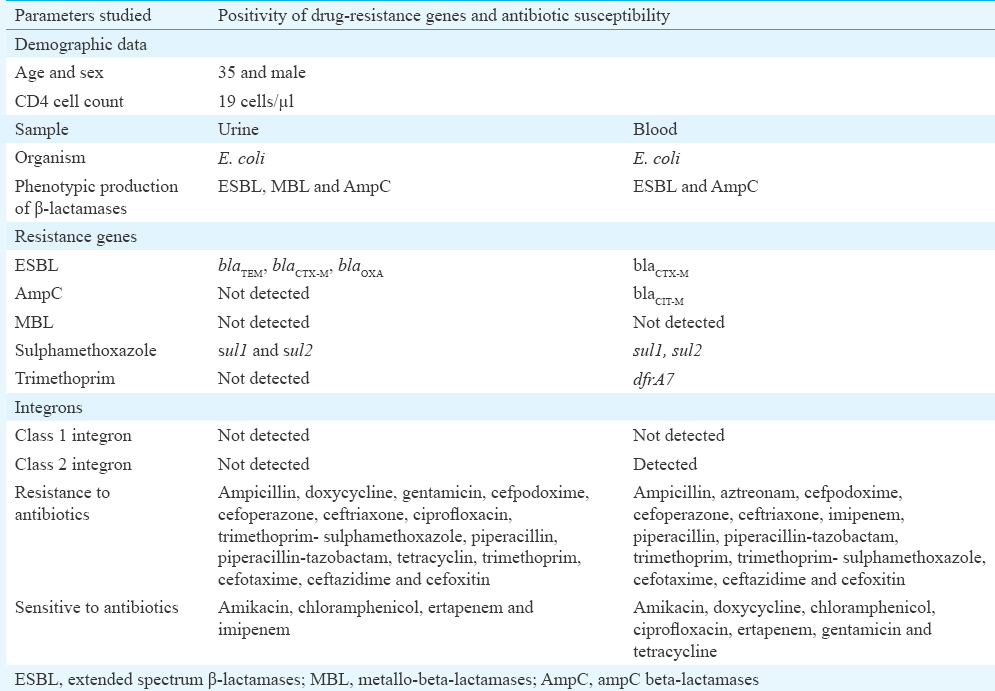
In this study, E. coli isolate from urine sample of HIV positive patient was found to be resistant to at least any one antibiotic of the classes β-lactams, aminoglycosides, tetracyclines, quinolones, pyrimidines and sulphonamides. It was also observed that the E. coli isolate from urine was phenotypically positive for ESBL, MBL and AmpC production. Vignesh et al14 reported that 80.6 per cent of the E. coli isolates from urine specimens from HIV patients were multidrug resistant and among them 83.3 per cent showed resistance to TMP-SMX, 94.4 per cent to ampicillin and 100 per cent sensitivity to imipenem and 44.4 per cent sensitivity to amikacin. They also reported that 25 per cent of the isolates showed positive for β-lactamase production. Phe et al15 found that antibiotic resistant E. coli was the main contributor of bloodstream infection in HIV patients which corroborated this finding. In this study, E. coli isolate from urine sample was positive for blaTEM, blaCTX-M and blaOXA genes related to ESBL production, and these findings were also in line with Lin et al16 who reported the coexistence of two or more ESBL genes in about 40 per cent of E. coli isolates. In our previous study17, we reported that Gram-negative bacteria harbouring β-lactamases-producing genes along with TMP-SMX resistance, and Class 1 and Class 2 integrons might make the treatment to bacterial infections more complicated in clinical settings. The probable source for the urinary tract and bloodstream infections of the HIV patient in this study may be from his own gut flora. An earlier study from India, reported that the endogenous translocation of gut flora was one of the major causes of infections of the urinary tract and bacteraemia18.
In conclusion, the present study showed the clonally different E. coli isolates causing blood and urinary tract infections in HIV patient from India and also the isolates harboured multiple drug-resistance genes. In this study, it is demonstrated that differences in antibiotic resistance and susceptibility profile of clonally different E. coli isolates causing different infections in an HIV patient may affect the selection of proper antibiotics for their treatment. This study also suggests that for effective treatment of bacterial infections, the proper antibiotic susceptibility testing should be carried out even for two bacterial isolates belonging to the same genus and species and isolated from two different infection sites of a patient.
Acknowledgment:
The Authors acknowledge Dr Srivani Ramesh, Assistant Professor, Department of Microbiology, Dr. ALM Post Graduate Institute of Basic Medical Sciences, Chennai, India for providing assistance in the molecular studies.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Emergence of multidrug resistant bacterial infection in HIV/AIDS cases. Health. 2012;3:49-52.
- [Google Scholar]
- Optimization of RAPD for fingerprinting Salmonella. Lett Appl Microbiol. 1997;24:243-8.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Document M100-S23 (23rd ed). Wayne, PA: Clinical and Laboratory Standards Institute; 2013.
- Mechanisms of decreased susceptibility to cefpodoxime in Escherichia coli. Antimicrob Agents Chemother. 2002;46:3829-36.
- [Google Scholar]
- Multiple CTX-M-type extended-spectrum beta-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in Northern Italy. J Clin Microbiol. 2003;41:4264-9.
- [Google Scholar]
- Multiplex PCR for rapid detection of genes encoding acquired metallo-beta-lactamases. J Antimicrob Chemother. 2007;59:321-2.
- [Google Scholar]
- Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002;40:2153-62.
- [Google Scholar]
- Integron content of extended-spectrum-beta-lactamase-producing Escherichia coli strains over 12 years in a single hospital in Madrid, Spain. Antimicrob Agents Chemother. 2005;49:1823-9.
- [Google Scholar]
- Susceptibility of Danish Escherichia coli strains isolated from urinary tract infections and bacteraemia, and distribution of sul genes conferring sulphonamide resistance. J Antimicrob Chemother. 2002;50:513-6.
- [Google Scholar]
- Genetic analysis of antibiotic-resistance determinants in multidrug-resistant Shigella strains isolated from Chilean children. Epidemiol Infect. 2005;133:81-6.
- [Google Scholar]
- Standard and real-time multiplex PCR methods for detection of trimethoprim resistance dfr genes in large collections of bacteria. Clin Microbiol Infect. 2007;13:1112-8.
- [Google Scholar]
- When whole-genome alignments just won't work: kSNP v2 software for alignment-free SNP discovery and phylogenetics of hundreds of microbial genomes. PLoS one. 2013;8:e81760.
- [Google Scholar]
- Extended-spectrum β-lactamase/AmpC-producing uropathogenic Escherichia coli from HIV patients: Do they have a low virulence score? J Med Microbiol. 2013;62:345-51.
- [Google Scholar]
- Urinary infections due to multi-drug-resistant Escherichia coli among persons with HIV disease at a tertiary AIDS care centre in South India. Nephron Clin Pract. 2008;110:c55-7.
- [Google Scholar]
- Does HIV status affect the aetiology, bacterial resistance patterns and recommended empiric antibiotic treatment in adult patients with bloodstream infection in Cambodia? Trop Med Int Health. 2013;18:485-94.
- [Google Scholar]
- Genotypic detection and molecular epidemiology of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a regional hospital in central Taiwan. J Med Microbiol. 2010;59:665-71.
- [Google Scholar]
- Dissemination of trimethoprim-sulfamethoxazole drug resistance genes associated with class 1 and class 2 integrons among gram-negative bacteria from HIV patients in South India. Microb Drug Resist. 2017;23:602-8.
- [Google Scholar]
- Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: A cohort study. Lancet Glob Health. 2016;4:752-60.
- [Google Scholar]





