Translate this page into:
Therapeutic implications of nano-encapsulated rifabutin, azithromycin & ethambutol against experimental Mycobacterium avium infection in mice
For correspondence: Dr Sadhna Sharma, Department of Biochemistry, Postgraduate Institute of Medical Education & Research, Chandigarh 160 012, India e-mail: sadhnabiochem@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Mycobacterium avium causes atypical infection in both immunocompetent and immunocompromised individuals. Conventional chemotherapy for M. avium infection is not efficient due to lengthy course of treatment and drug-associated toxic side effects. The present study was aimed at reducing dosing frequency of antimicrobial regimen consisting of azithromycin (AZM), rifabutin (RBT) and ethambutol (EMB) by encapsulation of drugs in nanoparticles (NPs) in experimental M. avium infection in mice.
Methods:
Poly (DL-lactide-co-glycolide) NPs containing anti-M. avium drugs were prepared, characterized and studied for their pharmacokinetics and pharmacodynamics parameters. Drug-loaded NPs were further analyzed for their therapeutic efficacy against experimental M. avium infection in mice.
Results:
Drug-loaded NPs were of size 227.3±16.4 for RBT, 334.35±11.7 for AZM and 509.85±20.5 for EMB with smooth surface morphology and negative zeta potential. AZM, EMB and RBT from NPs were detectable for 6, 4 and 5 days, respectively, in the mice plasma, whereas free drugs were cleared from mice circulation within 24 h. Chemotherapeutic effects of weekly administered drug-loaded NPs were equivalent to daily administered free drugs.
Interpretation & conclusions:
Our findings showed that NPs gave sustained release of drugs inside plasma and organs, thus decreasing dosage frequency, and their weekly dosage had therapeutic efficacy equivalent to daily dosage of free drugs.
Keywords
Azithromycin
ethambutol
Mycobacterium avium
nanoparticles
poly (DL-lactide-co-glycolide)
rifabutin
Mycobacterium infections were mostly attributed to Mycobacterium tuberculosis, but other species of Mycobacterium causing clinical disease have also been identified1. Mycobacterium avium is one such organism that belongs to Runyon Group III non-photochromogenic Mycobacterium2. Although M. avium infections can occur throughout the body, three types of clinical manifestations, viz. pulmonary disease, lymphadenitis and soft tissue infections, are most common34. On dissemination, non-tuberculous mycobacterial infections lead to fever, weight loss, sweating, diarrhoea, generalized lymphadenopathy, disseminated skin lesions and hepatosplenomegaly5. Immunocompromised patients, solid organ transplant recipients and aged individuals are at the highest risk of contracting disseminated infections as opportunistic infection56. M. avium is ubiquitously present in drinking water, household plumbing, peat-rich soils, brackish marshes and drainage water and hence has higher chance of infecting humans and spreading the disease7.
Macrolide drugs such as azithromycin (AZM) and clarithromycin are main drugs given for M. avium lung infections. However, these agents cannot be given in isolation due to the possibility of development of drug resistance8. Macrolide (AZM or clarithromycin) in combination with rifampin or rifabutin (RBT) and ethambutol (EMB) with or without an intravenous aminoglycoside are given in drug therapy. Duration of the antimicrobial therapy commonly exceeds 18 months or more. This therapeutic regimen has many limitations. Long duration of therapy, suboptimal efficacy and drug toxicity further lead to discontinuation of therapy by patients.
To eradicate the pathogens efficiently, an antibiotic should be carried in a form that is able to be endocytosed by phagocytic cells and then release it into these cells. The ultimate goal in drug therapy is the development of drug carriers that are capable of delivering drug(s) to their pharmacological sites of action and in a controlled rate appropriate to the disease9. With the development of sustained release drug formulation, the outcome and adherence to therapy can be improved which well further lead to reduction in number of times a drug has to be administered, resulting in better patient compliance. Further, the side effects of the drugs can thus be reduced which will further improve patient compliance. Drug delivery systems hold great promise in overcoming the limitations of conventional treatment regimens by increasing the bioavailability of drugs, reducing drug-associated toxicity and dosing schedules. Drug delivery systems based on liposomes, microparticles and nanoparticles (NPs) have been developed to deliver various types of drugs for different diseases10. NPs have emerged more efficient than liposomes and microparticles with respect to encapsulation efficiency and sustained release of antimicrobial agents. Poly (DL-lactide-co-glycolide) (PLGA) is an FDA-approved biodegradable and biocompatible drug carrier for human11. It is known to deliver antimicrobial drugs to treat various infectious diseases by improving bioavailability and pharmacokinetic profile of these agents1213. Encapsulation of antitubercular drugs has been reported to hold promise for better management of the disease1415. In this study, PLGA has been used to encapsulate most commonly recommended anti-M. avium drugs, i.e. azithromycin (AZM), EMB and RBT, and their pharmacokinetic and therapeutic potential in free and PLGA-NPs form against experimental M. avium infection in mice has been evaluated.
Material & Methods
PLGA Resomer 502 was purchased from Boehringer Ingelheim Pharma (Germany), polyvinyl alcohol (87-90% hydrolyzed, average molecular weight 30,000-70,000 Da) was purchased from Sigma (St. Louis, MO, USA). Rifabutin (RFB) was purchased from Shanghai Running Chemical Co. Ltd. RM Shanghai, China CN; Azithromycin from M/S Anuh Pharma Ltd., Mumbai, and Ethambutol was obtained from Sigma Chemical Co., USA. All other chemicals and reagents were obtained from standard companies.
Animals: Swiss albino mice of either sex weighing 20-25 g were obtained from the Central Animal House, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh. Animals were provided standard pellet diet and water ad libitum. The study was approved by the Institutional Animal Ethics Committee, PGIMER, Chandigarh, and was conducted in the department of Biochemistry, PGIMER, Chandigarh, India.
Culture: M. avium 1723 was obtained from Microbial Type Culture Collection (MTCC), Institute of Microbial Technology (IMTECH), Chandigarh, originally obtained from NCTC 8551, London, and maintained in the laboratory on Lowenstein-Jensen medium (HiMedia, Mumbai) and modified Youman's medium prepared in the laboratory1.
Preparation of PLGA-NPs: Drug-loaded PLGA-NPs were prepared by the multi-emulsion and solvent evaporation technique1 with slight modifications. PLGA-NPs of hydrophobic drugs, i.e. RBT and AZM, were prepared by single emulsion solvent evaporation technique (drug:polymer ratio of 1:1 for RBT, w/w and 1:10 for AZM, w/w) while PLGA-NPs of hydrophilic drug, i.e. EMB, were prepared by double emulsion method (drug:polymer ratio of 1: 2 (w/w). The NPs were recovered by centrifugation at 21,000 rpm (448,000 × g) for 20 min at 4°C, washed thrice with double distilled water and vacuum dried. PLGA-NPs were re-suspended in normal saline before each experiment.
Nanoparticle (NP) characterization: PLGA-NPs were characterized for their size and polydispersity index on Zetasizer 1000 HS (Malvern Instruments, Malvern, UK) based on photon correlation spectroscopy. The NPs containing drugs were lysed in 5 per cent sodium dodecyl sulphate in 0.5 N sodium hydroxide to release the drugs. The percentage drug encapsulation efficiency was determined as [amount of drug (mg) released from NPs/amount of drug (mg) initially taken to prepare the NPs] × 100. The drug-loading capacity was expressed as amount of drugs (mg) entrapped per gram of the polymer.
The drugs were analyzed by high-performance liquid chromatography (HPLC) comprising a dual-piston reciprocating pump, an online degasser, a UV-visual dual wavelength detector (each of series 200) and a 600 Series Link Interface for data acquisition/processing, all from PerkinElmer Instruments LLC (Shelton, CT, USA).
USP isocratic program using C18 columns (Symmetry® from Waters, USA; 4.6×250 mm; 5 μm particle size) was employed to analyze AZM, RBT and EMB1617. Analytical sensitivity of the method for AZM, RBT and EMB was found to be 0.3 μg/ml, 5 ng/ml and 0.5 μg/ml, respectively, and plasma recovery was 75.40, 90.4 and 80 per cent, respectively. Surface morphology of the drug-loaded PLGA-NPs was monitored by a scanning microscope (Philips XL 30 Netherlands).
In vivo drug disposition studies: Swiss albino mice were divided into the following groups (8 animals per group) - Group I, free AZM; Group II, RBT free; Group III, EMB free; Group IV, NPs encapsulating AZM; Group V, NPs encapsulating RBT; Group VI, NPs encapsulating EMB; Group VII, AZM, RBT and EMB in free form; Group VIII, NPs encapsulating AZM, RBT and EMB.
Drug doses were prepared as per body surface area of mice and were given at their therapeutic doses, i.e. AZM 500 mg/day, RBT 300 mg/day and EMB 1050 mg/day. For dissolution of hydrophobic drugs, dimethyl sulphoxide was used and hydrophilic drugs were dissolved in double distilled water. Drugs were dissolved immediately before time of administration, and 100-200 μl of the same containing therapeutic dose of the respective drug was given orally through gavage to mice. For the dosage of PLGA-encapsulated drugs, the lyophilized powder of PLGA-NPs was suspended in double distilled water, and 100-200 μl of the same containing required therapeutic dose was given orally through gavage.
The animals were bled at several time points (0.5, 1, 2, 3, 4, 6, 9, 12 and then every 24 h from day 1 to day 7) and plasma was isolated. For the analysis of RBT and AZM, 250 μl of plasma was deproteinized with methanol. For analysis of EMB, plasma was deproteinized with acetonitrile. The protein-free filtrate was used for the analysis of drugs by HPLC. In addition, 3-5 animals were sacrificed at different time intervals (day 1 to day 7) to monitor drug levels in tissue homogenates of lungs, liver and spleen.
Pharmacokinetic analysis: Various pharmacokinetic parameters were calculated using drug levels in plasma at particular time interval. Cmax, maximum drug concentration in plasma Tmax, time after administration of a drug when Cmax is reached; Kel, elimination rate constant (the rate at which a drug is removed from the system); t1/2, time required for elimination of half of the drug administered in body; AUC0-∞, area under the plasma drug concentration-time curve and reflects the actual body exposure to drug after administration of a dose of the drug and mean residence time (MRT) (h) were calculated using data analysis tools and Sigma Plot software (Version 8.0, Systat Software Inc., USA). The pharmacodynamic parameters such as Cmax/MIC and AUC/MIC (MIC is minimum inhibitory concentration of drug) ratios were also assessed.
Experimental infection and chemotherapy: Mice were intravenously infected with 0.1 ml of M. avium bacterial suspension containing approximately 1×107 colony forming units (cfu). Establishment of infection was checked by sacrificing six animals and plating lung, liver and spleen homogenates (dilutions 1:10, 1:100, 1:1000) on Middlebrook 7H11 agar supplemented with OADC for the enumeration of basal colony units.
Infected animals were divided into different groups with 12 animals in each group as indicated below: Group I consisted of untreated M. avium-infected animals; Group II was administered combination of free AZM, RBT and EMB orally; Group III was administered combination of AZM, RBT and EMB encapsulated in PLGA-NPs orally. Drugs were given at therapeutic doses (AZM 500 mg/day, RBT 300 mg/day, EMB 1050 mg/day) orally through gavage. Further, the free drugs were administered daily for four and eight weeks while the PLGA-NP-encapsulated anti-M. avium drugs were administered every sixth day a week (based on in vivo drugs release studies in plasma and organs). Control animals received phosphate buffer saline only. Drug preparations were made fresh daily. After four and eight weeks, i.e. 28 days and 56 days of chemotherapy, six animals at each time interval were sacrificed aseptically under sodium pentothal anaesthesia (48 h after end of therapy to prevent carryover effect of drug) from all three groups, and various organ (lung, liver and spleen) homogenates were prepared in one ml of sterile normal saline; 50 μl of undiluted and 1:10 diluted tissue homogenates (prepared in normal saline) were plated on Middlebrook 7H11 agar plates. The plates were incubated for 30 days post-inoculation at 37°C and log cfu were enumerated. The cfu counts in the spleen, liver and lungs were assessed for the effectiveness of the therapeutic regimens followed in this study.
Histopathological studies: Histopathological changes in organs of the mice subjected to chemotherapy with NPs encapsulating anti-M. avium drugs were monitored microscopically. Organs were fixed in 10 per cent formalin. The samples were transferred to a cassette and immersed in multiple baths of progressively more concentrated ethanol, to dehydrate the tissue, followed by toluene or xylene and finally extremely hot paraffin. The processed tissue was then taken out of the cassette and set in a mold and embedded. Then, a rotary microtome was used for thin (2-7 μm) sections of the tissues. The sections were processed for routine haematoxylin and eosin staining or acid-fast bacilli staining and observed under a microscope at ×100 and ×200 magnifications.
Statistical analysis: The pharmacokinetic or pharmacodynamic data among free drugs and PLGA-NP-encapsulated drugs were analysed using analysis of variance (ANOVA) followed by multiple comparison (post-hoc test), and multiple correction Bonferroni was applied. For cfu data comparison among various groups, ANOVA was used which was followed by Student's t test to compare individual treatment groups with each other and untreated control. All values used for animal studies were mean±standard deviation.
Results
Physicochemical characterization of drug-loaded PLGA-NPs: The characterization of PLGA-NPs is depicted in Table I. The single/double emulsion solvent evaporation technique resulted in NPs with fairly good drug encapsulation efficiencies, polydispersity index and zeta potential values, indicating a stable nanoformulation. Fig. 1(i) A,B,C shows the particle size distribution of PLGA-NPs encapsulating RBT, AZM and EMB. Scanning electron micrographs of NPs encapsulating RBT, AZM and EMB indicate their smooth surface morphology [Fig. 1 (ii) A,B,C].
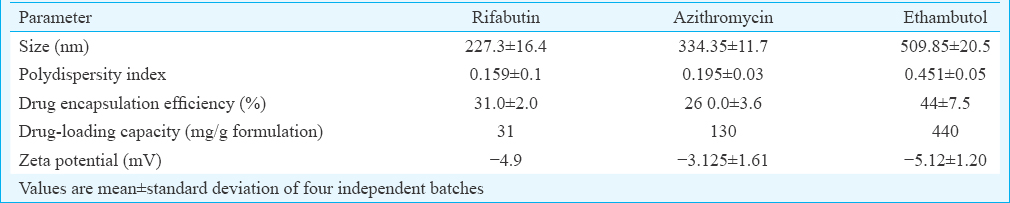
- (i) Particle size distribution of poly (DL-lactide-co-glycolide) nanoparticles encapsulating (A) rifabutin, (B) azithromycin and (C) ethambutol. (ii) Scanning electron micrograph of poly (DL-lactide-co-glycolide) nanoparticles encapsulating (A) rifabutin, (B) azithromycin, (C) ethambutol.
In vivo drug disposition studies: On single oral dose administration of drug-loaded PLGA-NPs in mice, RBT was detected in plasma from six hours onwards till seventh day, AZM was found to be present in plasma for four days and got cleared on day 5 and EMB got cleared from the circulation in plasma up to six days. The free drugs were cleared from the blood circulation within 24 h as depicted in Fig. 2. Following single oral dose administration of PLGA nanoformulations of RBT, AZM and EMB, the drugs were present at therapeutic concentrations in lungs, spleen and liver till day 7, 6 and 6, respectively. However, the free drugs stayed in various organs up to 24 h (in case of RBT and EMB) and up to 48 h for AZM (Fig. 3).
![In vivo plasma concentration time profile of orally administered (A) free rifabutin (RBT) and rifabutin-encapsulated poly (DL-lactide-co-glycolide) nanoparticles [poly (DL-lactide-co-glycolide) rifabutin nanoparticles] PLGA-RAT-NPs, (B) free azithromycin (AZM) and azithromycin-encapsulated nanoparticles [poly (DL-lactide-co-glycolide) azithromycin nanoparticles] PLGA-AZM NPs, (C) free ethambutol (EMB) and ethambutol nanoparticles [poly (DL-lactide-co-glycolide] ethambutol nanoparticles) PLGA-EMB NPs. Values are mean±standard deviation of three animals.](/content/175/2018/147/6/img/IJMR-147-594-g003.png)
-
In vivo plasma concentration time profile of orally administered (A) free rifabutin (RBT) and rifabutin-encapsulated poly (DL-lactide-co-glycolide) nanoparticles [poly (DL-lactide-co-glycolide) rifabutin nanoparticles] PLGA-RAT-NPs, (B) free azithromycin (AZM) and azithromycin-encapsulated nanoparticles [poly (DL-lactide-co-glycolide) azithromycin nanoparticles] PLGA-AZM NPs, (C) free ethambutol (EMB) and ethambutol nanoparticles [poly (DL-lactide-co-glycolide] ethambutol nanoparticles) PLGA-EMB NPs. Values are mean±standard deviation of three animals.

- (i) Organ (lung, liver, spleen) profile of rifabutin (RBT) (A) free form after 1 day, (B) poly (DL-lactide-co-glycolide)-encapsulated form after 3, 5, 7 days following single oral does administration to mice. (ii) Organ profile of azithromycin (AZM) in free (A) and poly (DL-lactide-co-glycolide)-encapsulated poly (DL-lactide-co-glycolide), (B) groups following single oral does administration to mice. (iii) Organ profile of ethambutol (EMB) in (A) free, (B) poly (DL-lactide-co-glycolide)-encapsulated in mice following single does administration. Animals were sacrificed at different intervals and organs were processed for drug analysis. Values are mean±standard deviation of three animals.
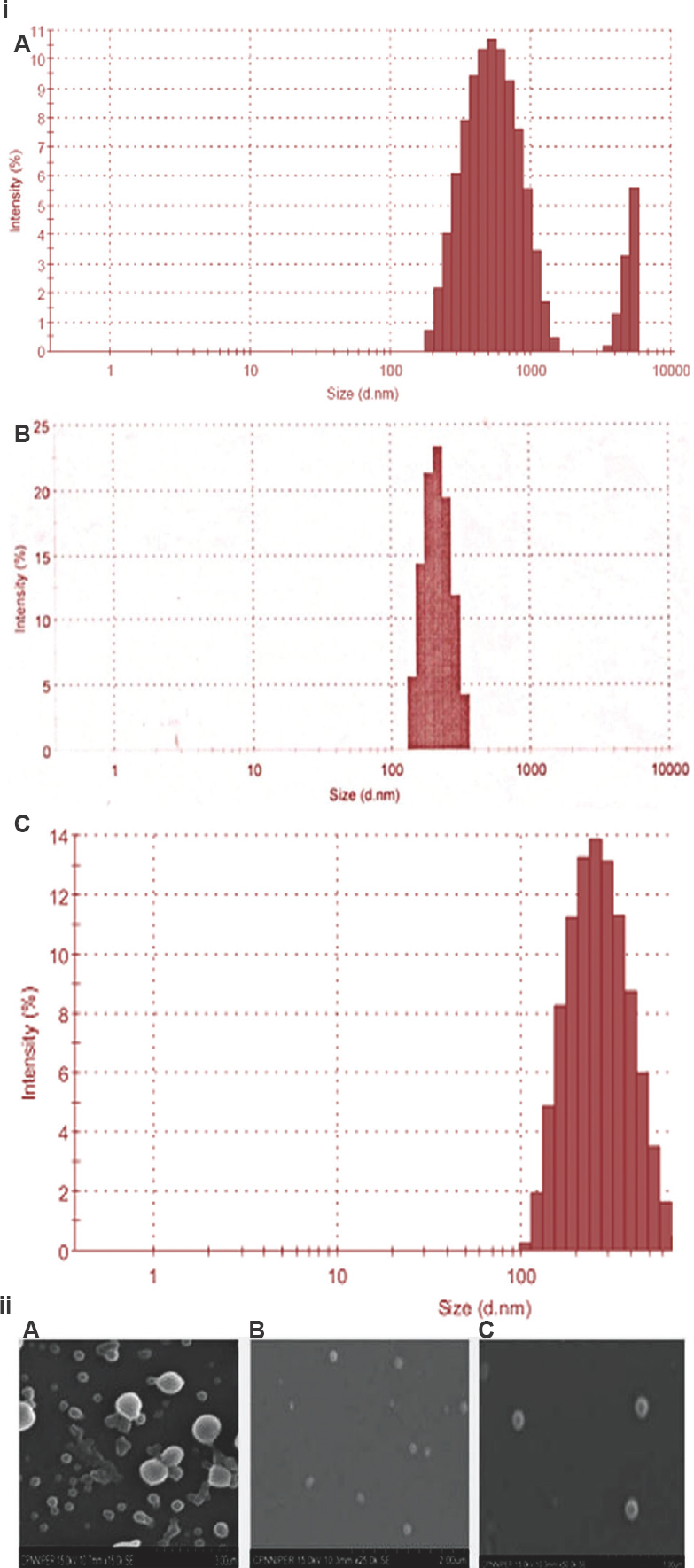
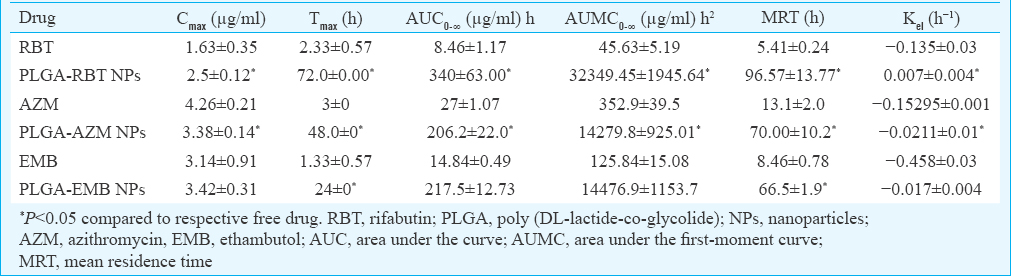
Pharmacokinetics: Encapsulating anti-M. avium drugs in PLGA-NPs have significantly improved their plasma profiles and hence enhanced their bioavailability as depicted by the significant increase in Cmax, (except for EMB), Tmax, AUC0-∞, AUMC0-∞, MRT and Kel of encapsulated drugs following oral administration in PLGA-NP form as compared to free drugs (Table II), thereby supporting the sustained release of the PLGA-NP formulations. The AUC24/MIC ratio of PLGA-NPs loaded RBT, AZM and EMB was found to be significantly higher than the free drugs, respectively. The Cmax/MIC ratio was higher in case of PLGA-NPs encapsulating RBT and EMB in comparison to free drugs, respectively. However, in case of PLGA-NPs loaded AZM, the Cmax/MIC ratio was decreased (Table III).
Chemotherapeutic efficacy: Oral administration of PLGA-NPs loaded anti-M. avium drugs after every sixth day a week resulted in approximately 1.54-, 1.48- and 1.3-fold clearance of bacilli after four weeks and 2.7-, 2.6-, 2.62-fold clearance of bacilli after eight weeks as compared to untreated controls from lungs, liver and spleen [Fig. 4(i) A,B]. Infected mice treated with oral free drugs daily for four and eight weeks showed comparable bacterial clearance to the PLGA-NP-treated animals.
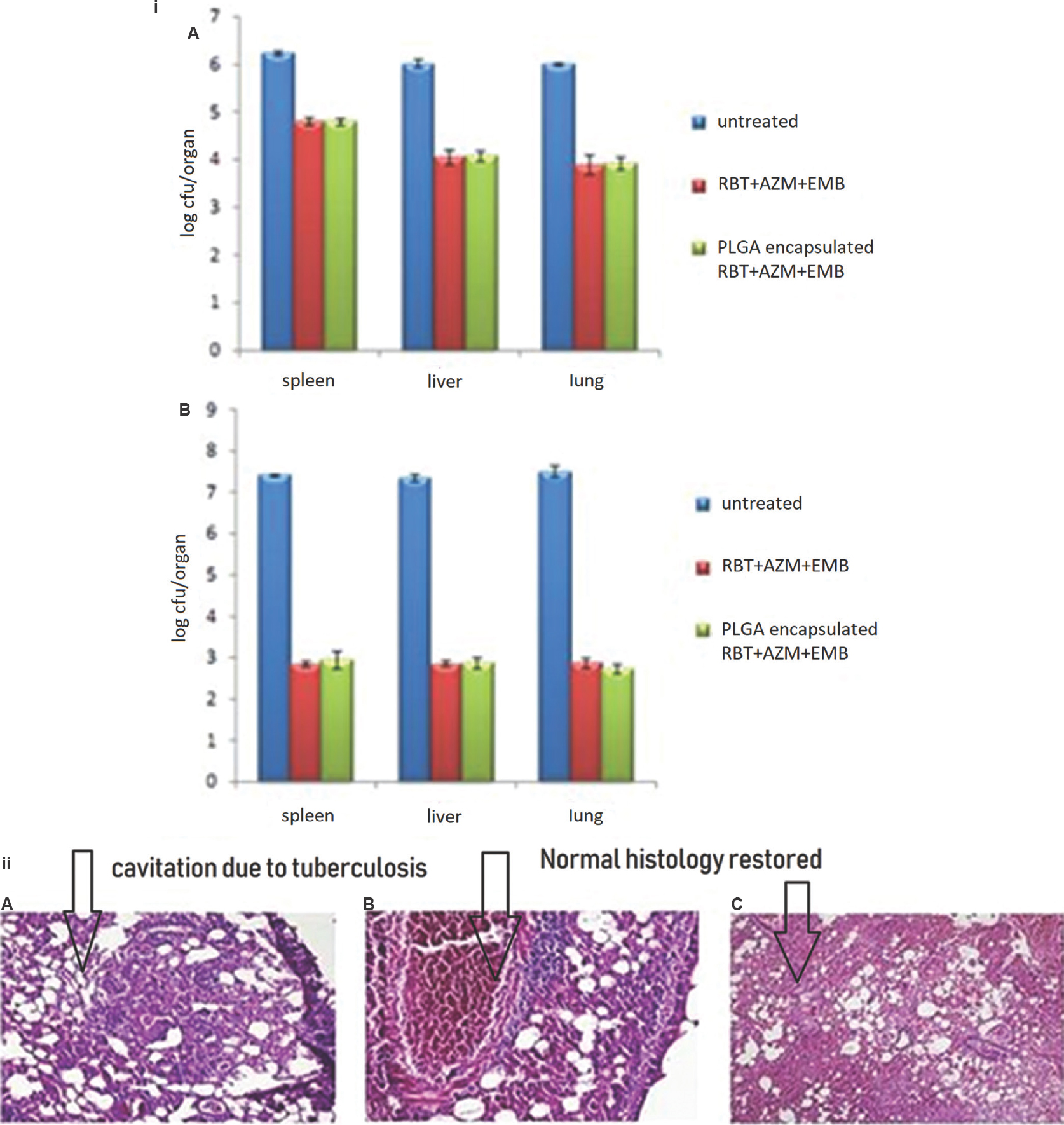
- (i) Chemotherapeutic efficacy of Mycobacterium avium drugs in free or poly (DL-lactide-co-glycolide) nanoparticle-encapsulated form against Mycobacterium avium infections after (A) four weeks and (B) eight weeks of chemotherapy. Values are mean±standard deviation of three animals. (ii) Lung H&E histopathology after eight weeks of chemotherapy: (A) untreated groups (100 ×), (B) free drugs group (200 ×) and (C) poly (DL-lactide-co-glycolide) nanoparticle-encapsulated drugs group (100 ×).
Histopathological studies: Untreated mice after eight weeks of infection showed granuloma in lung tissue along with lymphocytic clusters and congestion. Animals treated for eight weeks with AZM, RBT and EMB in the free form exhibited normal lung histology along with the presence of lymphocytes and congestion in blood vessels. By contrast, PLGA-NP-treated mice exhibited quite normal lung without the presence of lymphocytes and congestion [Fig. 4 (ii) A,B,C].
Discussion
The primary aim of this study was to develop a sustained release system for AZM, RBT and EMB using PLGA as drug carrier to treat experimental M. avium infection. PLGA is a biocompatible as well as biodegradable polymer which has been used extensively for improving the action of chemotherapeutics1819. In humans, phase II clinical trial have been done with doxorubicin containing PLA-PEG (polyethylene glycol NPs in prostate cancer and non-small cell lung carcinoma20. Both hydrophilic and hydrophobic drugs have been encapsulated in PLGA which can be administered orally. Furthermore, it has been shown that because of the bioadhesive properties of PLGA and its binding to mucosa of gastrointestinal tract, the residence and drug absorption time can be increased21. Pharmacokinetic and pharmacodynamic parameters of NPs are dominated by their physicochemical properties such as particle size, surface charge and shape of particles. Adhesion of NPs to biological cells and their interaction depends on the size of the particles22. Size of NPs has been known to influence drug loading, drug release and stability of NPs23. In this study, PLGA-NPs of size 227.3±16.4 for RBT, 334.35±11.7 for AZM and 509.85±20.5 for EMB with smooth surface morphology and fairly negative zeta potential were developed. Small particle size has been found to help in cellular uptake in oral delivery of the NPs24. PLGA-NPs loaded with anti-M. avium had negative zeta potential to minimize nonspecific NP interactions and aggregation25. In this study, hydrophobic drugs RBT and AZM showed higher drug encapsulation than hydrophilic drug EMB which can be attributed to the lipophilicity that prevents partitioning into the aqueous phase and hence increases drug entrapment into NPs during NP formulation26. PLGA-NPs showed a sustained release profile of anti-M. avium drugs, i.e. RBT, AZM and EMB, in the plasma for 6, 4 and 5 days, respectively, following a single oral administration. However, free RBT and EMB were found to be cleared from plasma within 24 h only, whereas free AZM was cleared within 48 h. A significant increase in Tmax, AUC0-∞, AUMC0-∞ and MRT as compared to the free drugs was observed for nano-encapsulated drugs. The increase in the elimination rate constant (Kel) of nano-encapsulated drugs as compared to free drugs supports the sustained release behaviour of the anti-M. avium drugs from PLGA-NPs. The increase in AUC0-∞ of anti-M. avium drugs upon encapsulation in PLGA-NPs may be explained by the prolonged release of drugs from NPs in the blood as well as decreased clearance of drug from circulation. A significant increase in MRT of anti-M. avium drugs loaded in PLGA-NPs as compared to free (unbound) drugs could be due to a change in the absorption and elimination rate of these drugs upon encapsulation in PLGA-NPs and also because of higher biodegradation time of the latter27. These observations are supported by other studies done in mice following oral administration of drugs loaded in PLGA-NPs282930. Effective anti-mycobacterial effect is expected from these formulations because of their superior PK/PD profile as compared to free drugs. Prolonged residence of anti-M. avium drugs in various organs formed the basis of the chemotherapeutic schedule. It was found that PLGA-NP-encapsulated drugs given orally every sixth day a week proved to be equivalently efficacious to free drugs which were given on daily basis in a four- and eight-week therapy schedule. Four weeks of chemotherapy with anti-M. avium drugs (RBT, AZM and EMB) in free and PLGA-encapsulated form resulted in approximately 1.54-, 1.48- and 1.3-fold reduction in bacterial growth from lungs, liver and spleen, respectively, as compared to untreated controls. Eight weeks of chemotherapy with anti-M. avium drugs (RBT, AZM and EMB) in free and PLGA-encapsulated form further reduced the bacillary load which was related with the histopathological changes. PLGA-NP-encapsulated anti-M. avium drugs (4-8 doses) depicted an equivalent therapeutic effect as 28-56 doses daily administered oral free drugs. Other research findings were in agreement with our results showing that effect of encapsulated drugs against M. avium infection was better than free drug therapy31. Our study had the limitation of studying immunocompetent disease model instead of immunocompromised model which could actually mimic the M. avium infections.
In conclusion, this study indicates that therapy with nanodrugs holds great potential in improving therapy against M. avium infection. NP-mediated delivery of anti-avium drugs can be further developed to achieve the clinical use of the therapy. It can help to cut down the frequent dosing intervals, reduce the toxic effects of drugs as well as prevent the drug resistance.
Financial support & sponsorship: The first author (TK) acknowledges the Indian Council of Medical Research, New Delhi, India for financial support.
Conflicts of Interest: None.
References
- Mycobacterium avium complex Mandell, Douglas and Bennett's principles and practice of infectious diseases. (4th ed). p. :2250-64.
- [Google Scholar]
- An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
- [Google Scholar]
- Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin Respir Crit Care Med. 2013;34:110-23.
- [Google Scholar]
- Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: An emerging public health disease. Am J Respir Crit Care Med. 2010;182:977-82.
- [Google Scholar]
- The pathophysiology of disseminated Mycobacterium avium complex disease in AIDS. J Infect Dis. 1999;179(Suppl 3):S461-5.
- [Google Scholar]
- Ecology of nontuberculous mycobacteria - Where do human infections come from? Semin Respir Crit Care Med. 2013;34:95-102.
- [Google Scholar]
- A randomized, double-blind trial comparing azithromycin and clarithromycin in the treatment of disseminated Mycobacterium avium infection in patients with human immunodeficiency virus. Clin Infect Dis. 2000;31:1245-52.
- [Google Scholar]
- Pegylated poly(lactide) and poly(lactide-co-glycolide) nanoparticles: Preparation, properties and possible applications in drug delivery. Curr Drug Deliv. 2004;1:321-33.
- [Google Scholar]
- A special issue on reviews in nanomedicine, drug delivery and vaccine development. J Biomed Nanotechnol. 2014;10:1635-40.
- [Google Scholar]
- Development of nanoparticles for antimicrobial drug delivery. Curr Med Chem. 2010;17:585-94.
- [Google Scholar]
- Development and characterization of folate anchored saquinavir entrapped PLGA nanoparticles for anti-tumor activity. Drug Dev Ind Pharm. 2015;41:1888-901.
- [Google Scholar]
- Curdlan-conjugated PLGA nanoparticles possess macrophage stimulant activity and drug delivery capabilities. Pharm Res. 2015;32:2713-26.
- [Google Scholar]
- Gelatin nanocarriers as potential vectors for effective management of tuberculosis. Int J Pharm. 2010;385:143-9.
- [Google Scholar]
- Mannosylated nanoparticulate carriers of rifabutin for alveolar targeting. J Drug Target. 2009;17:777-87.
- [Google Scholar]
- A new HPLC method for azithromycin quantitation. J Pharm Biomed Anal. 2002;27:833-6.
- [Google Scholar]
- Determination of rifabutin in human plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Appl. 1996;676:125-30.
- [Google Scholar]
- Nanomedicines for cancer therapy: State-of-the-art and limitations to pre-clinical studies that hinder future developments. Front Chem. 2014;2:69.
- [Google Scholar]
- Potential use of polymeric nanoparticles for drug delivery across the blood-brain barrier. Curr Med Chem. 2013;20:2212-25.
- [Google Scholar]
- Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4:128ra39.
- [Google Scholar]
- Poly (lactide-co-glycolide) particles of different physicochemical properties and their uptake by Peyer's patches in mice. Eur J Pharm Biopharm. 2005;61:1-3.
- [Google Scholar]
- Preparation and characterization of insulin nanoparticles using chitosan and its quaternized derivatives. Nanomedicine. 2008;4:115-20.
- [Google Scholar]
- Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31:3657-66.
- [Google Scholar]
- The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol Pharm. 2009;6:659-68.
- [Google Scholar]
- Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev Ind Pharm. 1999;25:471-6.
- [Google Scholar]
- Inhibition of HIV-1 in cell culture by oligonucleotide-loaded nanoparticles. Pharm Res. 2001;18:1096-101.
- [Google Scholar]
- Development of azithromycin-PLGA nanoparticles: Physicochemical characterization and antibacterial effect against Salmonella typhi. Colloids Surf B Biointerfaces. 2010;80:34-9.
- [Google Scholar]
- Oral poly(lactide-co-glycolide) nanoparticle based antituberculosis drug delivery: Toxicological and chemotherapeutic implications. Indian J Exp Biol. 2006;44:459-67.
- [Google Scholar]
- Nanoparticle encapsulated antitubercular drugs as a potential oral drug delivery system against murine tuberculosis. Tuberculosis. 2003;83:373-8.
- [Google Scholar]
- Efficacy of rifabutin-loaded microspheres for treatment of Mycobacterium avium-infected macrophages and mice. Drug Deliv. 2007;14:119-27.
- [Google Scholar]






