Translate this page into:
The IRF5 rs2004640 (G/T) polymorphism is not a genetic risk factor for systemic lupus erythematosus in population from south India
For correspondence: Dr Vir Singh Negi, Department of Clinical Immunology, Jawaharlal Institute of Postgraduate Medical Education & Research, Puducherry 605 006, India e-mail: vsnegi22@yahoo.co.in
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Genetic aberrations disrupting toll-like receptor and interferon homeostasis enhance the risk of systemic lupus erythematosus (SLE). Raised serum interferon-alpha (IFN-α) levels in SLE patients have been ascribed to polymorphism (rs2004640 G/T) in interferon regulatory factor 5 (IRF5) gene, resulting in enhanced transcript splicing. A positive association between IRF5 polymorphism and SLE risk has been reported in many populations. This study was aimed to find out frequency of IRF5 rs2004640 G/T polymorphism in patients with SLE and healthy controls and to assess its influence on susceptibility, clinical and serological characteristics of SLE.
Methods:
IRF5 rs2004640 (G/T) polymorphism was analyzed in 300 SLE patients and 460 age and sex matched controls by real-time PCR.
Results:
The IRF5 rs2004640 (G/T) polymorphism did not confer risk of SLE or influence clinical or serological phenotype. However, the mutant allele conferred a borderline risk to develop thrombocytopenia (odds ratio: 2.05, 95% confidence interval: 0.97–4.3, P=0.06) in patients with SLE.
Interpretation & conclusions:
Our study revealed that the IRF5 rs2004640 polymorphism was not a risk factor for SLE in population from south India. It may, however, be a useful genetic marker for thrombocytopenia in SLE patients. Although we could not demonstrate susceptibility toward lupus in the presence of IRF5 rs2004640 (G/T) polymorphism, further exploration of the genetic variability of IRF5 may help uncover its pathogenic role in Indian SLE patients.
Keywords
Autoantibodies
interferon-alpha
interferon regulatory factor 5
polymorphism
systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a complex autoimmune disease characterized by production of pathogenic autoantibodies against nuclear antigens. The aetiology of SLE is partly understood; however, genetic and environmental factors play a key role in determining the susceptibility, course and outcome of the disease. Higher incidence of disease within the family members supports the pivotal part of genetics with the pathogenesis of SLE. These aberrant genetic risk factors have a complex interplay and facilitate the development of autoimmunity by disrupting (i) immune cell signalling, (ii) immune complex disposal, and (iii) augmenting the type I interferon synthesis1. Advances in human genetics and gene expression studies have helped in understanding the immunopathogenesis of SLE2. Cytokines are the major transducers of immune signals and play a major role in autoimmune pathogenesis. One such cytokine is interferon alpha (IFN-α), a pleiotropic type I IFN with the potential to break immune tolerance, activation of autoreactive T and B cells and amplifying the autoimmune response. It was reported that interferon therapy augmented the secretion of autoantibodies in patients treated with IFN-α for non-autoimmune disorders34. The elevated serum IFN-α level corresponds with an elevated ‘IFN-α signature’ in peripheral blood mononuclear cells in lupus patients25.
The major pathogenic mechanism associated with the elevated secretion of IFN-α in SLE patient is as follows. Toll-like receptors (TLRs) and other pattern-recognition receptors present in the dendritic cells, upon recognizing the specific ligand and/or antigens in immune complexes enhance the secretion of IFN-α through the interferon regulatory factor 5 (IRF5) transcription system2. IRF5 is a transcription factor which induces the transcription of pro-inflammatory cytokines such as IFN-α, tumour necrosis factor-α (TNF-α), interleukin 12 (IL)-12 and IL-6 through MyD88-dependent activation of nuclear factor-κB (NF-κB) pathway6. The IRF5 polymorphisms have also been shown to be a genetic risk factor for other autoimmune diseases such as SLE7, scleroderma8, inflammatory bowel disease9, Sjögren's syndrome10 and rheumatoid arthritis11. Richez et al6 described the details of the variants in IRF5 gene, the molecular mechanism by which these genetic variants augment the secretion of IFN-α and mutant allele frequency in susceptible populations. One such genetic variant in IRF5 gene was the rs2004640 G/T polymorphism reported to be a genetic risk factor for SLE in Caucasian, Afro-American and in Asian ethnic population12131415161718. A recent study of three polymorphisms (rs10954213, rs2004640 and rs2280714) in IRF5 gene revealed that the GTA haplotype was a risk for SLE and the rs2004640 T allele was an independent risk factor for SLE and development of lupus nephritis in Egyptian children19. The rs2004640 G/T polymorphism was reported to alter the splicing process and lead to excessive production of IFN-α, which might augment the autoimmune responses through its pleiotropic effects on various immune cells67. Identifying the genetic modifications of critical molecules in the type I IFN pathway is expected to increase the understanding of disease pathogenesis and its impact on patients with SLE. This study was carried out to determine the frequency of IRF5 rs2004640 polymorphism in SLE patients and healthy controls among south Indian population and to analyze its influence on pathogenesis, clinical and autoantibody profile of lupus.
Material & Methods
This study was carried out in the Clinical Immunology OPD at Jawaharlal Institute of Postgraduate Medical Education & Research (JIPMER), Puducherry, India, during September 2009 to March 2012. The sample size for cases and controls was calculated using CaTS power calculator for genetic studies20 with a power of 0.8 and 0.05 as the significance level. A total of 300 SLE patients attending Clinical Immunology OPD, fulfilling the 1997 American College of Rheumatology criteria for SLE21 were included as cases. The disease activity in SLE patients was assessed and graded by systemic lupus erythematosus disease activity index22. A total of 460 age- and sex-matched volunteers with no known family history of autoimmune diseases, diabetes mellitus, malignancies hypertension and other co-morbidities were included as control population. The Institute Ethics Committee of JIPMER had approved the study. A signed written informed consent was obtained from all the participants.
Peripheral venous blood (5 ml) was collected from the participants and was used to extract genomic DNA by phenol-chloroform method following the published protocol23. This protocol involved selective osmotic lysis of RBCs, removal of haemoglobin by repeated washing and concentration of leucocytes. The DNA from the concentrated leucocytes was obtained by lysing the cells using hypertonic saline and proteinase K enzyme. Phenol-chloroform was added to localize the DNA in aqueous phase; later, the DNA was precipitated in absolute ethanol and dissolved in Tris EDTA buffer (pH 8.0). The DNA concentration was measured (Picodrop, Thermo Scientific, USA) and diluted to contain 50 ng/μl. The diluted DNA was used for genotyping protocols. The IRF5 gene rs2004640 G/T polymorphism was tested by TaqMan real-time genotyping assay using the primers and probes obtained from Applied Biosystems (CA, USA). The primer pairs used for IRF5 G/T genotyping were Forward-5’-CAGCTGCGCCTGGAAAG-3’ and Reverse 5’-GGGAGGCGCTTTGGAAGT-3’. The probe sequences used for detection of G and T alleles were VIC: TGTAGGCACCCCCCCG and FAM: TGTAGGCACCCACCCG, respectively.
Statistical analysis: The genotype and allele frequencies between controls and cases were compared using the Chi-square test with Yates's correction or Fisher's exact test. Relative risk conferred by the mutant allele was arrived by calculating the odds ratio (OR) and confidence interval 95 per cent (CI 95%). Age, gender, complement level, disease duration, organs involved, autoantibodies and IRF5 genotypes were used as covariates to perform the logistic regression analysis. Statistical analysis for this study was carried out using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
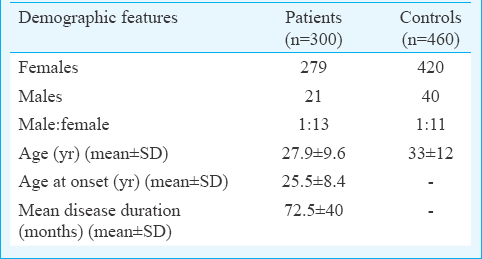
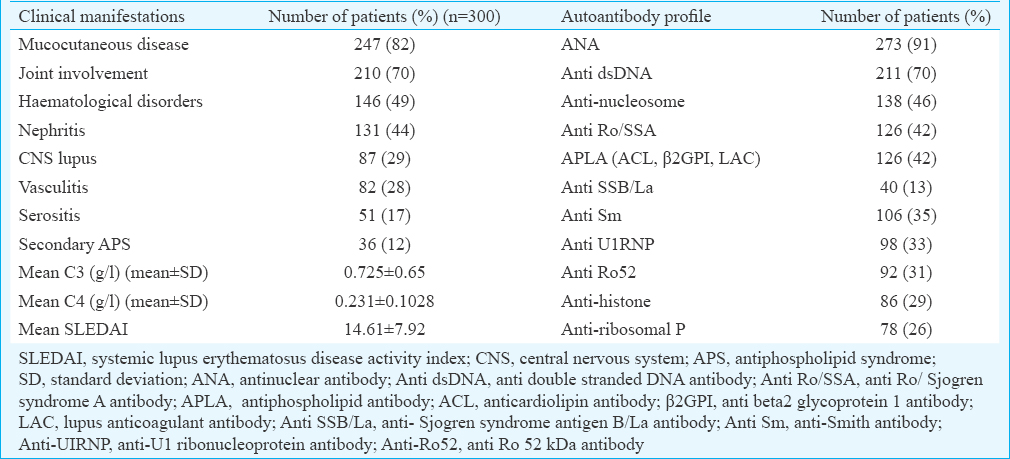
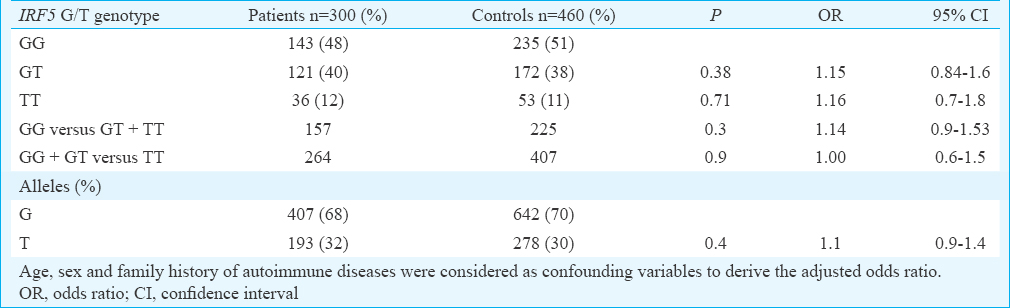
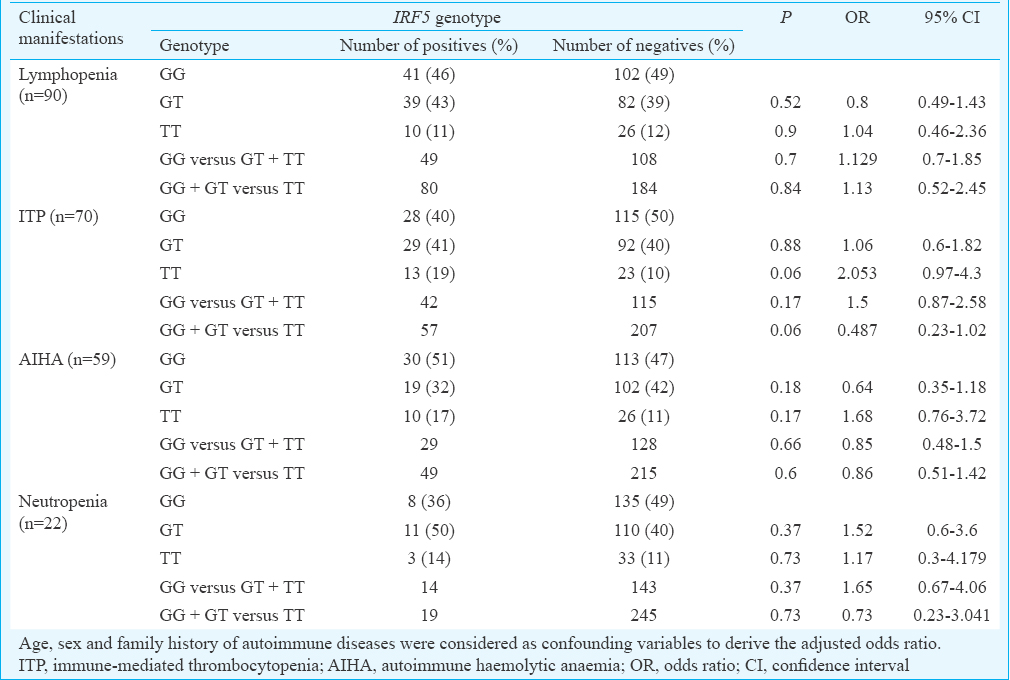
The demographic features of the cases and controls are depicted in Table I. Major clinical manifestations and the autoantibody reactivity are presented in Table II. The IRF5 rs2004640 C/T genotype and allele distribution amongst cases and controls are given in Table III. It was observed that the distribution of minor allele T was almost similar in patients (32%) and controls (30%). The homozygous and the heterozygous mutant genotypes were found to be almost equally distributed in both the cases and controls and thus did not reveal any significant risk to develop SLE. Analysis of influence of IRF5 rs2004640 G/T polymorphism on clinical phenotypes revealed a borderline association with the development of immune thrombocytopenia (ITP) in patients with homozygous mutant genotype (P=0.06, OR - 2.05, 95% CI=0.97–4.3) (Table IV).
Discussion
SLE is a multisystem autoimmune disorder characterized by the production of autoantibodies with an overwhelming immune response against self-antigens. A severe deviation from immune homeostasis is the classical feature of SLE which includes a compromised cytokine profile favouring only the amplification of the autoimmune response. An increased circulatory level of IFN-α is a prominent finding in active SLE and its rise in the serum is directly proportional to severity of the disease1224. In our control population, a 30 per cent prevalence of the minor allele frequency was observed, which was similar to that reported from China12, Japan13 and Korea14 but lesser than the reports of 50 per cent in Caucasians16171825 and African Americans26.
In this study, a marginally higher frequency of the mutant allele was observed in SLE patients in comparison to the controls (32 vs 30%); however, the difference was not significant. A similar negative association between the variant T allele with SLE was reported in the Chinese12 and Japanese13 but not in Koreans14 and a subgroup of Shandong Han Chinese population15. Although a significant association was observed in Koreans, the reported odds ratio was 1.32, which rendered their results ambiguous. In addition to the above SNP, analysis of three more variants, namely, the rs729302, rs752637 and rs2280714 did not appear to confer a significant risk14. The haplotypes constructed from these polymorphisms also failed to show a positive association with the development of SLE in Koreans14. In Caucasians, the IRF5 2004640 T allele was reported to confer a significant risk to develop SLE16171825, which was also replicated in many of the genome wide association studies (GWAS) studies conducted in Caucasians27. Kawasaki et al13 analyzed the IRF5 variants such as rs2004640, rs10954213, rs6953165, rs41298401 and rs11770589 in a Japanese cohort and found that none of these were associated with SLE susceptibility. However, when they combined their results with a Korean cohort, they noted a significant association with the development of SLE in Asians. They concluded that the polymorphism in Intron 1 of IRF5 gene played a crucial role in the expression of IFN pathway genes. Dang et al28 reported that the IRF5 2004640 polymorphism was not a risk for SLE in northern Han Chinese. They observed significant interaction and a higher incidence of mutant alleles of IRF5 (rs2004640) and STAT4 (rs7574865) variants in SLE patients.
In our study the patients with IRF5 rs2004640 mutant allele T showed a trendency to develop ITP. It was observed that a larger proportion of SLE patients manifesting ITP were carriers of IRF5 homozygous mutant genotype (19 vs 10%) (data not shown). Stratification of patients based on the clinical phenotype might have rendered the sample size low to obtain a significant association. Wazny and Ariano29 reported that the major side effect of IFN-α therapy was thrombocytopenia attributable to bone marrow suppression, immune-mediated destruction and platelet aggregation. Therefore, ITP in SLE patients might be due to the elevated secretion of IFN-α under the influence of IRF5 rs2004640 mutant allele.
Although the IRF5 rs2004640T variant allele was reported to be associated with the development of lupus nephritis in Chinese SLE patients28, we did not find any such association in our patients. The frequency of the anti-dsDNA and anti-Ro 52 antibodies with TT genotype was higher in patients versus controls, i.e. 14 per cent versus 9 per cent and 18 per cent versus 10 per cent, respectively; however, the difference was not significant which might be due to a small sample size (data not shown). Similar findings were also reported by Qin et al30 in patients with lupus nephritis. Niewold et al31 reported that four SNPs (rs2004640, rs3807306, rs10488631 and rs2280714) along with insertion polymorphisms in the promoter region and exon 6 of the IRF5 gene influenced the production of autoantibodies in SLE patients and in their family members from European ancestry. They also reported a few haplotypes which appeared to augment the production of IFN-α and conferred risk to develop SLE. They highlighted the differential effect of IRF5 genotype on serum IFNα activity that was detectable only in patients who were positive for either anti-ribonuclear protein antibodies (anti-Ro, La, Sm and RNP) or anti-dsDNA but was not seen in patients with higher background IFNα activity, who were positive for both autoantibodies. We did not observe any positive association between the rs2004640 mutant allele T and the production of autoantibodies (data not shown). It has been reported that the autoantigen-antibody complexes can themselves augment the IFN-α production through the endosomal TLR system. The plasmacytoid dendritic cells with the help of TLR 9 were capable of recognizing the dsDNA in immune complexes and trigger the production of IFN-α. The mechanism by which the IFN-α induces and amplifies the autoimmune responses has been described in detail32.
The major limitation of our study was that we analyzed IRF5 rs2004640 polymorphism only. Study of other major polymorphisms in the IRF5 gene would further help to establish the population-specific risk haplotype. In various studies, IRF5 pathway has been targeted by therapeutics directed at the endosomal TLRs and IFN-α333435. Thus, IRF5 genotype may help to differentiate between the responders and non-responder patients with respect to these therapies.
In conclusion, our study revealed that the IRF5 rs2004640 polymorphism was not a risk factor for SLE in population from southern India. However, with a larger sample size, it may emerge as a possible genetic marker to predict the development of thrombocytopenia in SLE patients. Screening the other polymorphisms in IRF5 gene will provide an insight into the role of IRF5 genetics in SLE pathogenesis.
Financial support & sponsorship: This study was funded by the ICMR-INSERM (Indian Council of Medical Research - Institut national de la santé et de la recherche médicale) through Grant no. INDO/FRC/604/08-08 and 50/9/2008/BMS and Department of Science and Technology, Government of India, New Delhi (Grant No. SR/SO/HS-67/2004 dated 03.08.2007).
Conflicts of Interest: None.
References
- Genetic studies of systemic lupus erythematosus in Asia: Where are we now? Genes Immun. 2009;10:421-32.
- [Google Scholar]
- Interferon regulatory factors in human lupus pathogenesis. Transl Res. 2011;157:326-31.
- [Google Scholar]
- Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J Intern Med. 1990;227:207-10.
- [Google Scholar]
- Systemic lupus erythematosus arising during interferon-alpha therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clin Rheumatol. 2005;24:178-81.
- [Google Scholar]
- Polymorphisms in the tyrosine kinase 2 and interferon regulatory factor 5 genes are associated with systemic lupus erythematosus. Am J Hum Genet. 2005;76:528-37.
- [Google Scholar]
- Role for interferon regulatory factors in autoimmunity. Joint Bone Spine. 2010;77:525-31.
- [Google Scholar]
- Interferon regulatory factors: Critical mediators of human lupus. Transl Res. 2015;165:283-95.
- [Google Scholar]
- Association between the IRF5 rs2004640 functional polymorphism and systemic sclerosis: A new perspective for pulmonary fibrosis. Arthritis Rheum. 2009;60:225-33.
- [Google Scholar]
- An insertion-deletion polymorphism in the interferon regulatory factor 5 (IRF5) gene confers risk of inflammatory bowel diseases. Hum Mol Genet. 2007;16:3008-16.
- [Google Scholar]
- The CGGGG insertion/deletion polymorphism of the IRF5 promoter is a strong risk factor for primary Sjögren's syndrome. Arthritis Rheum. 2009;60:1991-7.
- [Google Scholar]
- Association of a haplotype in the promoter region of the interferon regulatory factor 5 gene with rheumatoid arthritis. Arthritis Rheum. 2007;56:2202-10.
- [Google Scholar]
- Association of a haplotype of IRF5 gene with systemic lupus erythematosus in Chinese. J Rheumatol. 2008;35:360-2.
- [Google Scholar]
- Association of IRF5 polymorphisms with systemic lupus erythematosus in a Japanese population: Support for a crucial role of intron 1 polymorphisms. Arthritis Rheum. 2008;58:826-34.
- [Google Scholar]
- Replication of the genetic effects of IFN regulatory factor 5 (IRF5) on systemic lupus erythematosus in a Korean population. Arthritis Res Ther. 2007;9:R32.
- [Google Scholar]
- Relationship between polymorphism sites of IRF5, TLR-9 and SLE patients in Shandong Han population. Zhonghua Yi Xue Za Zhi. 2009;89:3038-42.
- [Google Scholar]
- Genetic association of IRF5 with SLE in Mexicans: Higher frequency of the risk haplotype and its homozygozity than Europeans. Hum Genet. 2007;121:721-7.
- [Google Scholar]
- Association of a common interferon regulatory factor 5 (IRF5) variant with increased risk of systemic lupus erythematosus (SLE) Ann Hum Genet. 2007;71:308-11.
- [Google Scholar]
- A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat Genet. 2006;38:550-5.
- [Google Scholar]
- Interferon regulatory factor 5 gene polymorphism in Egyptian children with systemic lupus erythematosus. Lupus. 2017;26:871-80.
- [Google Scholar]
- GAS Power Calculator. Available from: http://csg.sph.umich.edu/abecasis/cats/gas_power_calculator/index.html
- Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
- [Google Scholar]
- Derivation of the SLEDAI. A disease activity index for lupus patients. The committee on prognosis studies in SLE. Arthritis Rheum. 1992;35:630-40.
- [Google Scholar]
- A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
- [Google Scholar]
- Three functional variants of IFN regulatory factor 5 (IRF5) define risk and protective haplotypes for human lupus. Proc Natl Acad Sci U S A. 2007;104:6758-63.
- [Google Scholar]
- Interferon regulatory factor-5 is genetically associated with systemic lupus erythematosus in African Americans. Genes Immun. 2008;9:187-94.
- [Google Scholar]
- Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204-10.
- [Google Scholar]
- Gene-gene interactions of IRF5, STAT4, IKZF1 and ETS1 in systemic lupus erythematosus. Tissue Antigens. 2014;83:401-8.
- [Google Scholar]
- Evaluation and management of drug-induced thrombocytopenia in the acutely ill patient. Pharmacotherapy. 2000;20:292-307.
- [Google Scholar]
- Association of IRF5 gene polymorphisms and lupus nephritis in a Chinese population. Nephrology (Carlton). 2010;15:710-3.
- [Google Scholar]
- IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann Rheum Dis. 2012;71:463-8.
- [Google Scholar]
- The interferon-alpha signature of systemic lupus erythematosus. Lupus. 2010;19:1012-9.
- [Google Scholar]
- Targeting toll-like receptors: Emerging therapeutics? Nat Rev Drug Discov. 2010;9:293-307.
- [Google Scholar]
- Emerging therapies for systemic lupus erythematosus - Focus on targeting interferon-alpha. Clin Immunol. 2012;143:210-21.
- [Google Scholar]
- Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: A phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 2013;65:1011-21.
- [Google Scholar]






