Translate this page into:
Occurrence & predictors of osteoporosis & impact of body composition alterations on bone mineral health in asymptomatic pre-menopausal women with HIV infection
For correspondence: Dr Deep Dutta, Department of Endocrinology, Post Graduate Institute of Medical Education & Research & Dr. Ram Manohar Lohia Hospital, 1 Baba Kharak Singh Marg, New Delhi 110 001, India e-mail: deepdutta2000@yahoo.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Data on bone mineral density (BMD) and sarcopenia are scant from young females with HIV. This study was conducted to determine occurrence, predictors and impact of body composition alterations on osteoporosis in pre-menopausal women with HIV.
Methods:
A total of 214 females with serologically documented HIV infection were screened, of whom 103 pre-menopausal women, 25-45 yr age, clinically stable, having at least one year follow up data, underwent hormonal and dual-energy X-ray absorptiometry analysis for BMD and body composition. Seventy five matched controls were also evaluated.
Results:
Females with HIV had significantly lower BMD and Z-score at lumbar spine (LS), total femur, neck of femur (NOF), and radius ultra-distal (UD) compared to controls. Osteoporosis at least at one site was observed in 34.95 per cent patients, compared to eight per cent in controls (P <0.001). Most common site of osteoporosis in females with HIV was radius UD (24.27%), followed by radius 33 per cent (17.48%), radius total (15.53%) and greater trochanter, NOF and LS (6.80% each). HIV patients had significantly lower bone mineral content, lean mass (LM), fat per cent, android (A) fat, gynoid (G) fat, and A/G ratio. LM and fat mass (FM) were −15.65 and −11.54 per cent lower in HIV patients, respectively. Osteoporosis patients had significantly higher use of antiretroviral therapy and lower LM, FM and fat per cent. On logistic regression, LM followed by A/G ratio and BMI were the best predictors of osteoporosis. Sarcopenia was observed in 17.5 per cent patients.
Interpretation & conclusions:
Our results showed that osteoporosis and sarcopenia were significant problems in young women with HIV. HIV was associated with greater LM loss, which was critical for bone health. Sarcopenia may predict low BMD in HIV.
Keywords
Bone mineral density
fat mass
HIV
lean mass
osteopenia
osteoporosis
sarcopenia
With the increasing burden of HIV infection, coupled with better outcomes, endocrinopathies are increasingly being encountered in HIV patients1. Endocrinopathies are commonly associated with impaired bone health23. In a meta-analysis, patients with HIV had three times higher prevalence of low bone mineral density (BMD), compared to controls4. High prevalence of vitamin D deficiency has been observed in patients with HIV5. Most of the data on BMD in HIV are available from males, predominantly Caucasians6. Most of these studies are limited by lack of evaluation of representative controls6. Data from females are scant. BMD data in females are predominantly available from older women, which are confounded by post-menopausal osteoporosis7.
Alterations in body composition are known to have an impact on bone health in non-HIV-infected individuals8. Different patterns of alterations in lean mass (LM), fat mass (FM) and bone mineral content (BMC) have been reported in HIV9. Hence, this study was aimed to assess BMD at different sites and body composition parameters in young pre-menopausal women with HIV, to determine the occurrence and predictors and to evaluate impact of body composition alterations on osteoporosis in them.
Material & Methods
Consecutive ambulatory females, 25-45 yr age, having regular cycles or with oligomenorrhoea/menstrual irregularities, with serologically documented HIV infection, in stable clinical condition, without any acute, severe illness, attending the Antiretroviral Therapy (ART) clinic, Post Graduate Institute of Medical Education & Research & Dr. Ram Manohar Lohia Hospital, New Delhi, India, during August 2015 to July 2016, were considered. Severely ill patients with multiple co-morbid states, who would warrant hospital admission, were excluded. Patients with a history of hospital admissions in the last two months were also excluded. Post/peri-menopausal women or women with premature ovarian insufficiency [amenorrhoea greater than six-month duration and/or follicle-stimulating hormone (FSH) >40 mIU/ml] were excluded. Patient records were reviewed and those having clinical data of at least one year follow up were further evaluated. Patients with available CD4 cell counts at diagnosis and at first follow up (6-12 months after diagnosis) were included. The study protocol was explained, and only those who gave informed written consent were included. The institutional ethics committee approved the study protocol.
Data were collected regarding duration of HIV infection and details of highly active antiretroviral therapy (HAART). Data were also collected regarding the past or current evidence of opportunistic infections (bacterial, viral and fungal). Patients underwent detailed clinical assessment, including anthropometry. Patients were called subsequent day in fasting state for blood sampling. Blood samples (5 ml each) were collected in plain and ethylenediaminetetraacetic acid (EDTA) vacutainers (Becton Dickinson, USA). Serum was separated from the blood collected in a plain vacutainer and processed immediately for routine biochemical analysis, and one aliquot of serum was stored at −20°C for specific immunological (hormonal) assays. EDTA samples were processed for haematological analysis.
Chemiluminescent microparticle immunoassay (VITROS® ECiQ Immunodiagnostic System, Johnson & Johnson, USA) was used for the estimation of 25-hydroxy-vitamin-D (25OHD), intact parathyroid hormone, prolactin, testosterone, estradiol, luteinizing hormone (LH) and FSH. Serum 25OHD assay had analytical sensitivity of 8.0 ng/ml, analytical range of 8-150 ng/ml and intra- and inter-assay coefficient of variation (CV) of 3.4 and 5.5 per cent, respectively. Testosterone assay has analytical sensitivity of 0.16 μg/dl and analytical range of 4.90-2163 ng/dl with intra- and inter-assay CV of 3.8 and 7.4 per cent, respectively. LH assay has analytical sensitivity of 0.216 mIU/ml and analytical range of 0.216-200 mIU/ml with intra- and inter-assay CV of 8.8 and 11.3 per cent, respectively. FSH assay has analytical sensitivity of 0.66 mIU/ml and analytical range of 0.66-200 mIU/ml with intra- and inter-assay CV of 2.8 and 10.1 per cent, respectively. Serum calcium, phosphate, alkaline phosphate and renal function tests were done using clinical chemistry auto-analyzer based on dry chemistry micro-slide technology (VITROS® 350 Chemistry System, Johnson & Johnson, USA). CD4 cell count was performed using flow cytometry (Becton Dickinson Immunocytochemistry Systems, San Jose, CA, USA).
All patients underwent BMD assessment by dual-energy X-ray absorptiometry (DEXA; Discovery Wi Series, Hologic Inc., Waltham, MA, USA) at lumbar spine (LS, L1-L4 anteroposterior), left proximal femur [neck (NOF), greater trochanter (GT) & total femur (TF)] and left forearm regions [radius 33%, ultra-distal (UD) & radius total]. Quality control procedures were done as per the manufacturer's recommendations. The instrument was calibrated on a daily basis, using phantom provided by the manufacturer and the CV at different sites was found to be <1.0 per cent over the duration of the study. BMD of subjects was recorded in terms of absolute mineral content (in g/cm2) at various sites. Due to significant difference in age of patients in the study (25-45 yr), Z-score [number of standard deviation (SD) away from average value of age- and gender-specific reference group] was used to compare BMD across groups. Osteoporosis at any site was diagnosed if Z-score was < −2 SD10. T-score was not used for defining osteoporosis as it has been established for diagnosis of osteoporosis in post-menopausal state11.
DEXA was also used for estimation of whole-body BMC (kg) total body fat (kg), percentage FM (%), FM (kg), LM (kg), android (A) fat (kg) and gynoid (G) fat (kg). Body composition analyses of soft tissues were performed using the QDR2000 Product Software, version 7.10A (Hologic, USA). The lower border of android region was set at the upper border of pelvis. The upper border of android region was set at a level being 20 per cent of distance from the upper border of pelvis to neck. The upper border of gynoid region was set at level below the upper border of pelvis at a distance of 1.5 times the length of the android region. The lower border of the gynoid region was set at level at which the length of gynoid region was twice the length of android region11. Reproducibility of DEXA measurements was derived from the root mean square SD of two repeat measurements11. Technique precision for body composition variables was 12.32 g for BMC (0.96% CV), 166.3 g for LM (0.74% CV) and 156.2 g for FM (0.72% CV). Clinical, biochemical and DEXA parameters were also collected from 75 age-, sex- and BMI-matched healthy controls, who gave informed written consent and were recruited from nursing staff of the Institute.
Percentage skeletal muscle mass (PSMM) (total LM/weight × 100) was calculated in patients and controls for estimation of sarcopenia12. Due to lack of normative date from India population, as per the European Working Group on Sarcopenia in older people recommendations12, a PSMM of <2 SD below mean in healthy control group was used to define sarcopenia13.
Immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients is characterized by clinical deterioration secondary to re-establishment of immunity following HAART10. It is usually observed in patients with low baseline CD4 count, which increases rapidly following HAART initiation. HAART has been linked to increased systemic inflammation and autoimmunity. IRIS has been defined as CD4 count >200 cells/μl in patients who previously had CD4 counts <100-200 cells/μl10. Hence, patients in our study with baseline CD4 counts <200 cells/μl, which increased to >200 cells/μl at first follow up following HAART initiation, were defined to have IRIS.
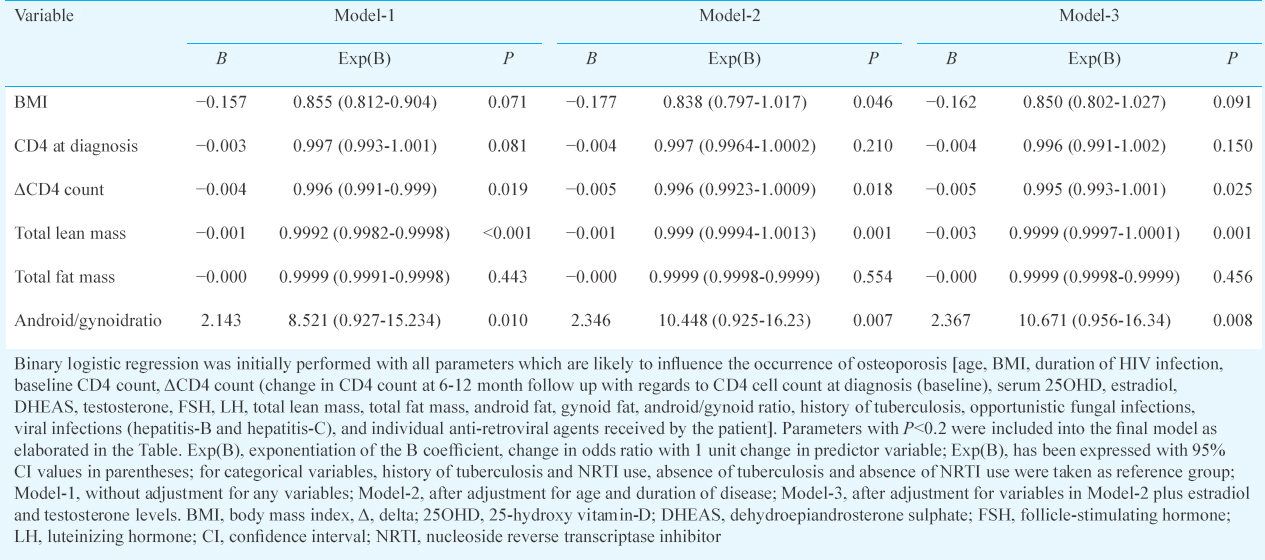
Statistical analysis: Normality of the distribution of variables was checked using the Kolmogorov-Smirnov test. Independent t test and Wilcoxon rank-sum test were done for normally distributed and skewed variables, respectively. Chi-square test was used for categorical variables. Pearson's or Spearman's correlation coefficient were calculated for normally and non-normally distributed variables respectively. Binary logistic regression was done to determine the factors that independently predict the occurrence of osteoporosis after adjusting for variables, in different models. Statistical Package for the Social Sciences (SPSS) version 20 (Chicago, IL, USA) was used for data analysis.
Results
A total of 214 consecutive females with HIV were screened, of whom 103 who fulfilled all criteria and gave consent underwent hormonal and DEXA analysis (Figure). In addition, 75 healthy age-, sex- and BMI-matched controls recruited from nursing staff of the hospital, who gave consent underwent same set of investigations. Median age of our patients with HIV infection was 35 yr, having median disease duration of 57 months with 91.26 per cent (n=94) on HAART, 37.86 per cent (n=39) having a history of tuberculosis, 34.95 per cent (n=36) having a history of IRIS and 85.44 per cent (n=88) patients having vitamin D deficiency/insufficiency (Table I). Females with HIV had significantly lower BMD and corresponding Z-score at LS, TF, NOF, and radius UD, as compared to controls (Table II). BMD at radius total was also significantly lower among HIV-infected females (P=0.002) (Table II). Z-score at radius, total was lower among HIV-infected females but did not reach statistical significance (Table II).
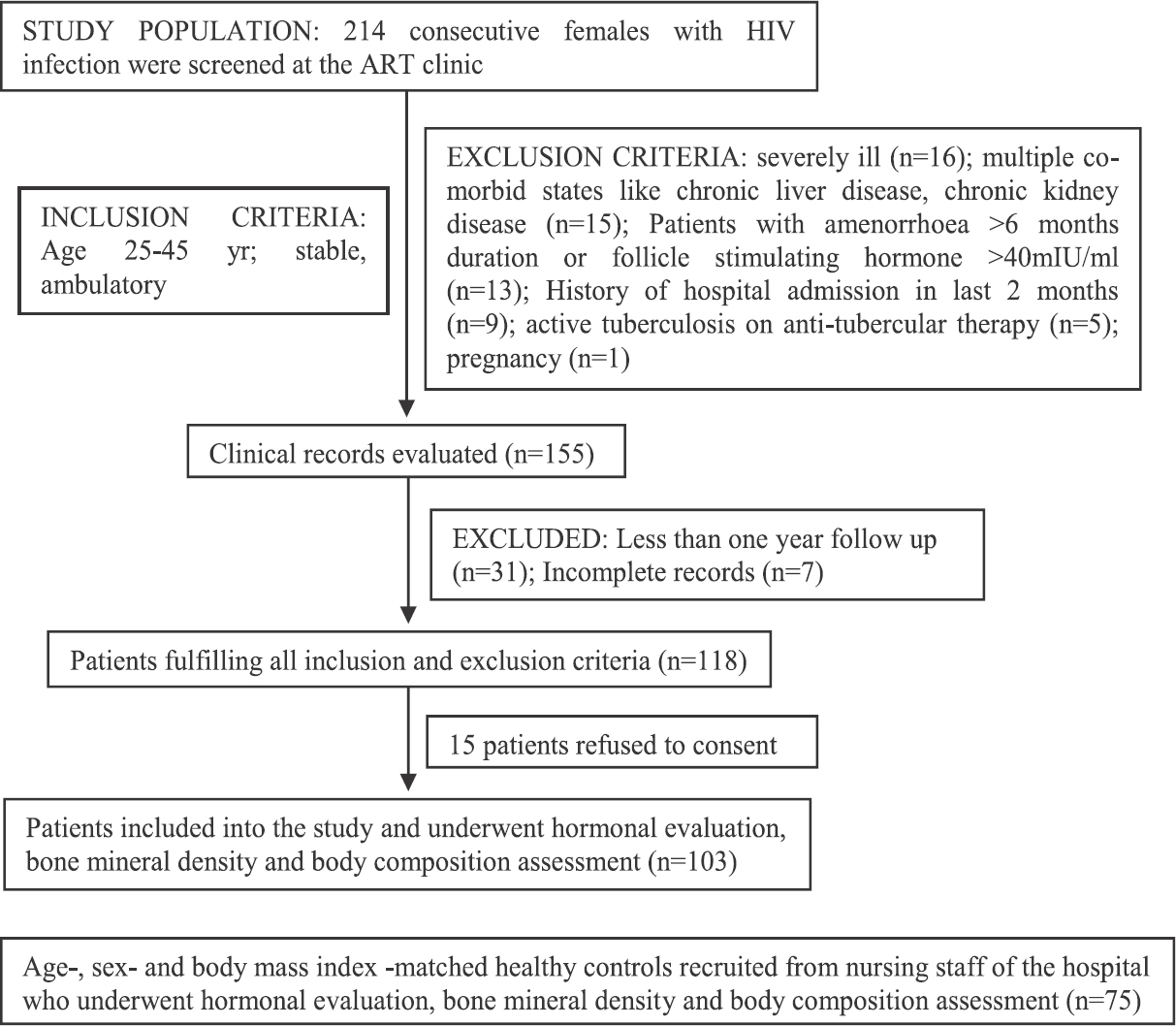
- Flowchart elaborating the study protocol.
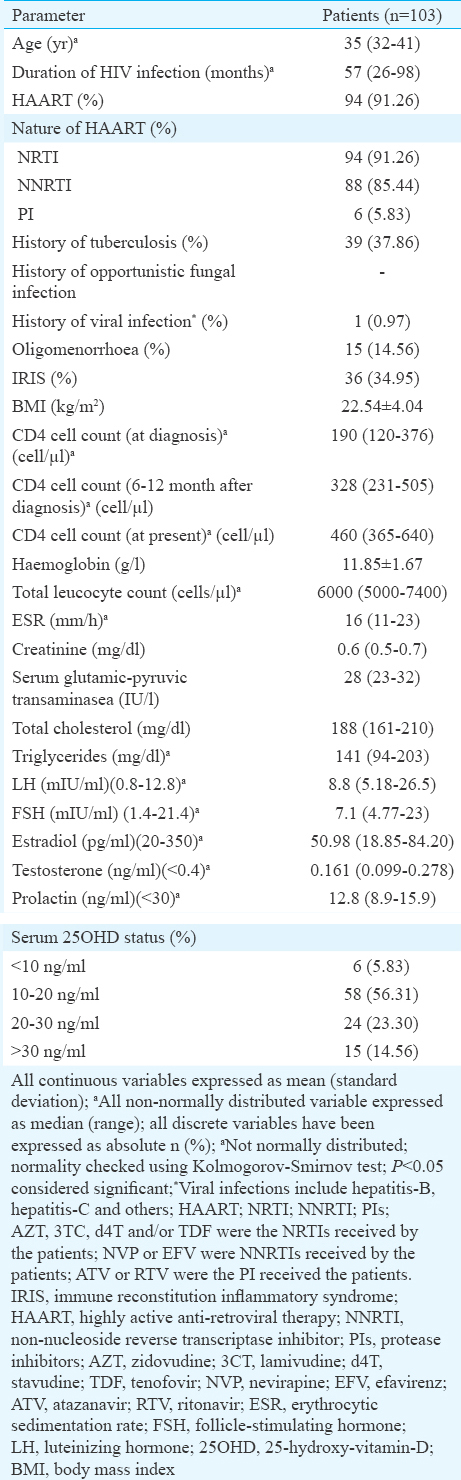
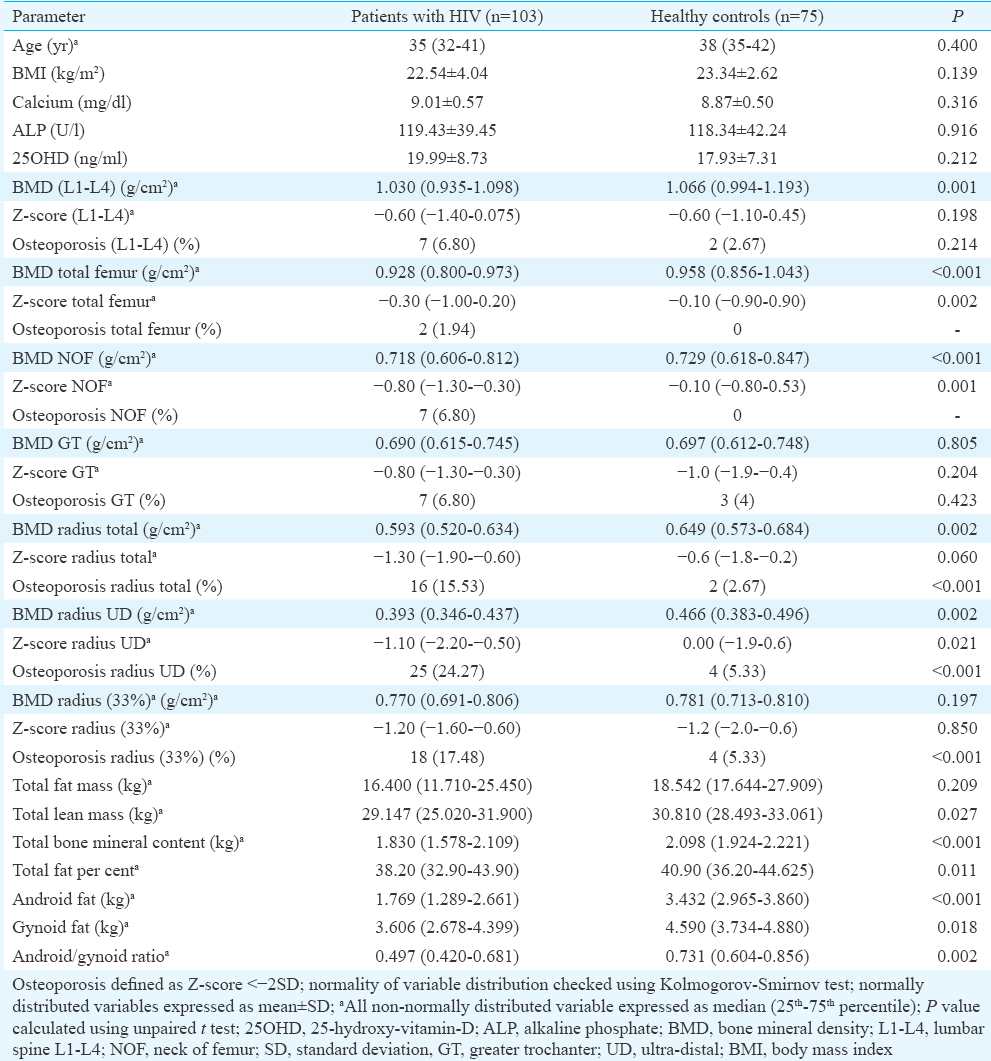
Osteoporosis involving at least any one site was observed in 36 (34.95%) females with HIV as compared to six (8.00%) individuals in controls (P <0.001). Most common site of osteoporosis among HIV-infected females was radius UD (24.27%; n=25), followed by radius 33 per cent (17.48%; n=18), radius total (15.53%; n=16) and GT, NOF and LS (6.80%; n=7 each) and was least frequent at TF (1.94%; n=2) (Table II). Patients with HIV had significantly lower total BMC (P<0.001), LM (P=0.027), total fat per cent (P=0.011), android fat (P<0.001), gynoid fat (P=0.018) and android/gynoid (A/G) ratio (P=0.002) as compared to controls (Table II). LM and FM were −15.65 and −11.54 per cent lower in HIV patients as compared to controls. Among females with HIV, those with osteoporosis had higher use of HAART (P<0.001), higher vitamin D levels (P=0.010), lower FM (P=0.034), LM (P<0.001) and total fat per cent (P=0.037) (Table III). In females with HIV, total LM and FM had significant positive correlation with BMD at different sites (Table IV). The correlations were stronger for LM as compared to FM. Gynoid fat had significant positive correlation with BMD at LS, total radius and radius 33 per cent in females with HIV (Table IV). Among controls, only BMD TF had significant positive correlation with LM (Table IV). A/G ratio had a negative correlation with BMD at all sites in both groups but statistically not significant (Table IV).
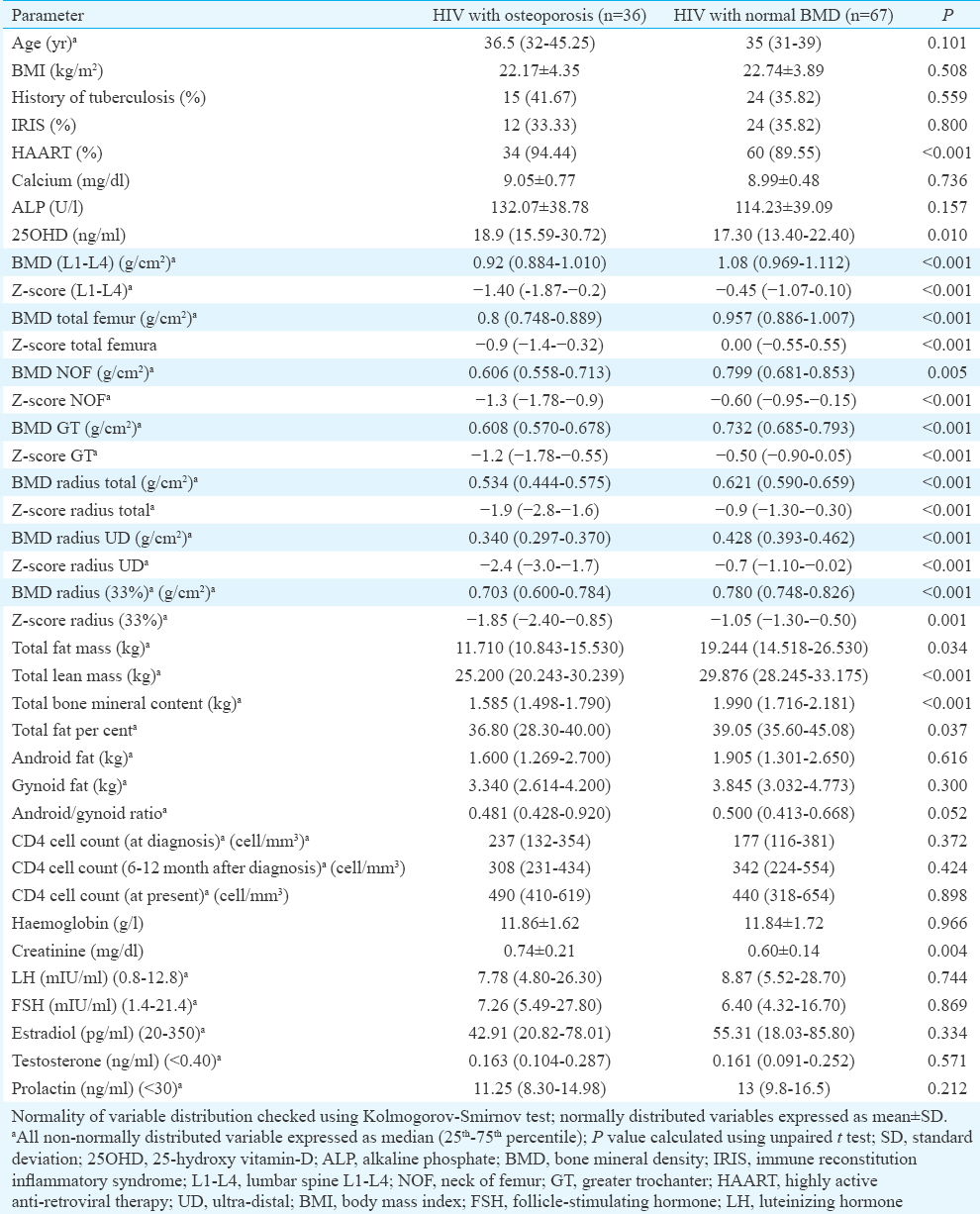
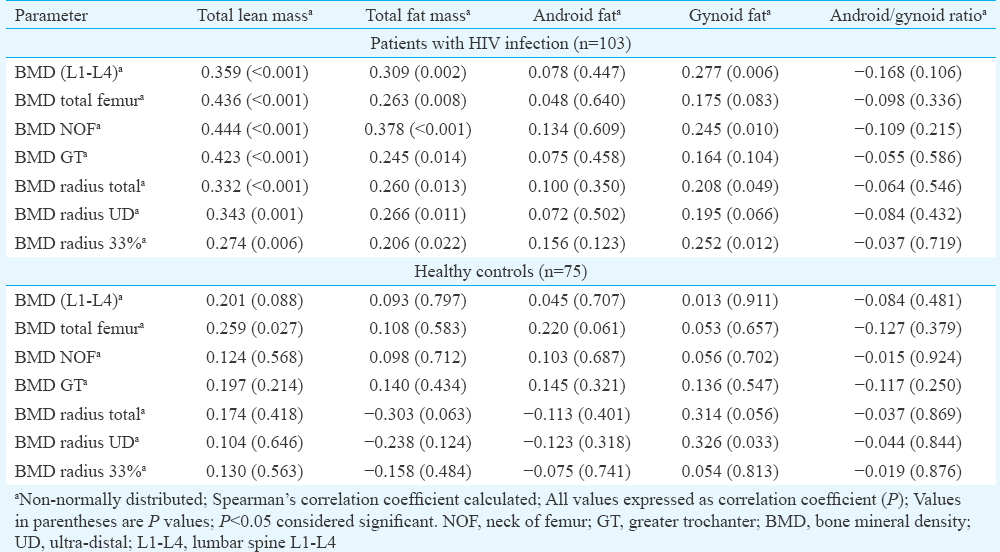
Binary logistic regression analysis revealed that LM and A/G ratio were consistently the best predictors of occurrence of osteoporosis in HIV (Table V). Increased LM was associated with decreased risk and increased A/G ratio was associated with increased osteoporosis risk, without adjusting for any variable (Model-1), after adjusting for age and disease duration (Model-2), and after adjusting for variables in Model-2 plus estradiol and testosterone (Model-3). Increased BMI was associated with decreased osteoporosis risk, which was significant after adjusting for age and disease duration (Model-2) (Table V).
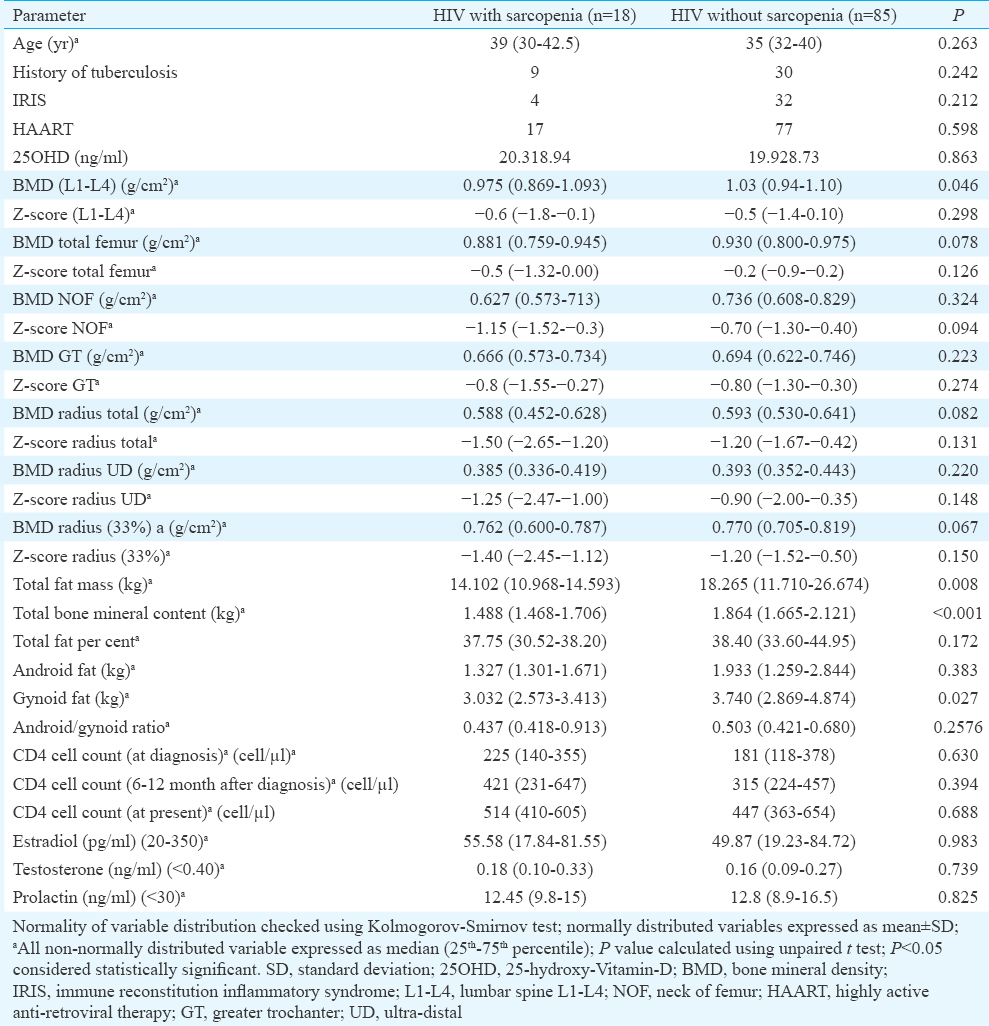
PSMM in controls and females with HIV was 56.78±6.63 per cent and 56.61±13.58 per cent, respectively. Sarcopenia was observed in 18 females with HIV infection and two in controls (P<0.001). HIV-infected patients with sarcopenia had significantly lower BMD LS, FM, BMC and gynoid fat (Table VI). BMD TF, radius total and radius 33 per cent were also lower in patients with sarcopenia, but did not reach statistical significance (Table VI). Thirty five per cent patients in the HIV group (n=103) had osteoporosis, in contrast to eight per cent in the control group (n=75). This evaluation achieved 99.5 per cent power, keeping type-I error (alpha) at 5 per cent.
Discussion
Bone mineral (BM) loss in HIV is multifactorial. HIV viral proteins (vpr and gp120) promote osteoclast activity, and p55-gag suppresses osteoblast activity, in vitro14. Malnutrition, underweight, physical inactivity, malabsorbtion, hypogonadism, glucocorticoids, vitamin D deficiency, substance abuse, smoking all contribute to BM loss in HIV13. HIV-infected patients in our study had a high occurrence of vitamin D deficiency/insufficiency (85.44%), reflective of high vitamin D deficiency state in general population15. Median 25OHD among HIV-infected patients in our study was significantly higher than in controls. The marginally higher 25OHD in HIV patients may be explained by vitamin D supplementation in some patients. Vitamin D deficiency apart from its classical role in calcium absorption and bone formation has been linked with increased systemic inflammation, which may further accentuate bone loss16.
Our study showed that low BMD and osteoporosis are a significant problem in young females with HIV, affecting 35 per cent of patients. BM loss in HIV is predominantly appendicular, with osteoporosis predominantly effecting different sites at the wrist.
Maximum data on osteoporosis in HIV-infected females are available from post-menopausal women, where a great variability in osteoporosis prevalence has been reported1718. In a study from Spain involving 671 HIV patients, 23 per cent had osteoporosis6. In contrast, in a cohort of 51 HIV patients (86% males; 30-54 yr age) from the USA, four patients (7.8%) had osteoporosis17. In a study from Turkey, 24 per cent HIV-infected patients had osteoporosis, having median age of 40.1 yr with 84 per cent being males18. Dravid et al19 reported 29.6 and 36.6 per cent HAART naïve (n=40) and on HAART patients (n=496) to have osteoporosis, which was similar to disease burden observed in our study.
BMI was an independent predictor of osteoporosis in our study, after adjusting for age and disease duration, in accordance with the previous reports45. Increased mechanical loading of bone secondary to increased BMI explains the positive impact on BMI on BMD8. In our study, HIV-infected females with osteoporosis were more likely to be receiving HAART, as compared to HIV-infected females without osteoporosis. However, regression analysis did not show any particular antiretroviral agent to be associated with increased osteoporosis risk. HAART in general is believed to contribute to osteoporosis1920. HAART is believed to induce marked BMD loss (2-6%) within first two years, irrespective of antiretroviral agent used21. IRIS may have a role in this early HAART-related bone loss22. However, in our study, occurrence of IRIS was not significantly different in HIV-positive females with osteoporosis as compared to those without. Protease inhibitors and tenofovir have been most commonly linked to BM loss22. Tenofovir, through proximal renal tubular toxicity, causes renal phosphate wasting, explaining BM loss22. Only two studies have evaluated fracture outcomes in HIV-infected peri-menopausal and post-menopausal women, with both observing significantly higher fracture rates (>1.5 times with regards to uninfected controls)22. HIV/HCV co-infection is a predictor of increased fractures22. No link was observed between sex steroids and gonadotropins levels with BMD in our study. In literature, there is a single report where a positive relation was observed between increased circulating estradiol with increased BMD in HIV-infected females21.
ΔCD4 cell count (change in CD4 cell count at 6-12 months after HAART initiation with regard to pre-HAART CD4 cell count levels) was consistently an independent predictor of osteoporosis after adjusting for variables in Models-1, 2 and 3. A greater ΔCD4 count was associated with decreased risk of osteoporosis. A greater ΔCD4 count may imply a better immune function, which may be associated with reduced circulating HIV viral load. However, HIV viral load (RNA levels) was not evaluated in this study and was a limitation. In our study, baseline CD4 count was not found to be a predictor of BMD loss. In contrast, Grant et al23 observed a lower baseline CD4 count to be associated with greater BMD loss in first two years following HAART.
HIV was associated with a greater decline in LM as compared to FM. Our study demonstrated that decreased LM (muscle mass) was the strongest and best predictor of osteoporosis in HIV. HIV-related muscle mass loss is multifactorial and may be due to myopathy, distal symmetric polyneuropathy or wasting syndrome1820. Decreased muscle mass (sarcopenia) was observed in 17.5 per cent of HIV-infected patients in our study. A few studies have also used decreased muscle strength and physical activity to define sarcopenia13. However, these were not evaluated in this study and was a limitation. Poor nutrition, increased systemic inflammation, hormonal alterations, neuromuscular and mitochondrial dysfunction are some of the factors responsible for sarcopenia in HIV2022. A study in males with HIV infection from Cleveland, USA, also documented a high prevalence of low BMD (68.2%) and sarcopenia (21.9%)24. In that study, loss of LM with accompanying increased in central fat accumulation and peripheral fat atrophy in HIV patients, as compared to controls24. Our study showed that after LM, increased central adiposity with peripheral fat loss (increased A/G ratio) was associated with increased osteoporosis in young females with HIV. This was in accordance with the previous report of association between increased abdominal visceral fat and reduced spine BMD in 41 HIV patients25.
Increased LM results in more mechanical loading of the body as compared to FM, resulting in a greater bone mass26. It has been suggested that bone adapts more to dynamic muscle load than to static load, explaining the stronger effect of LM over FM on BMD27. Isolated increase in FM results in lesser dynamic loading of bones secondary to decreased physical activity. This explains the beneficial effect of physical activity on bone health. Additional benefits of increased LM include prevention of falls, thus decreasing fracture risk. The term ‘sarco-osteoporosis’ is increasingly being used to highlight the impact of neuromuscular system on bone health and osteoporotic fractures28. There are limited data on therapeutic outcomes in HIV-associated osteoporosis. Apart from bisphosphonates, no other agent has been evaluated in HIV-associated osteoporosis. Alendronate over a period of 96 weeks has been documented to improve BMD in patients on HAART29. In another randomized controlled trial, two annual doses of 4 mg zoledronic acid were demonstrated to have a beneficial effect on BMD, which persisted for at least five years after the second dose30.
Limitations of this study included its cross-sectional design. The gonadotropins were not assessed in the early follicular phase, which was a limitation of this study. Further, visceral and subcutaneous fat could not be assessed separately as DEXA (not computed tomography) was used for body composition analysis. Strengths of this study included the relatively large homogenous group of young HIV-infected premenopausal woman with matched healthy controls.
In conclusion, this study highlighted the unrecognized significant burden of osteoporosis (35%) and sarcopenia (17.5%) in young premenopausal Indian women with HIV infection. This study also highlighted the importance of adequate LM in maintaining bone health. Evaluation of sarcopenia has been shown to have an important role in predicting low BMD and osteoporosis in HIV.
Acknowledgment
Authors acknowledge the staff of the department of Endocrinology, Radiology and Biochemistry for their assistance in making this work possible, and thank all the patients and nursing staff of the hospital for their support and cooperation.
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- Prevalence and predictors of thyroid dysfunction in patients with HIV infection and Acquired Immunodeficiency Syndrome: An Indian perspective. J Thyroid Res 2015 2015:517173.
- [Google Scholar]
- Severity and pattern of bone mineral loss in endocrine causes of osteoporosis as compared to age-related bone mineral loss. J Postgrad Med. 2016;62:162-9.
- [Google Scholar]
- Occurrence of osteoporosis & factors determining bone mineral loss in young adults with Graves’ disease. Indian J Med Res. 2015;141:322-9.
- [Google Scholar]
- Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: A meta-analytic review. AIDS. 2006;20:2165-74.
- [Google Scholar]
- Efavirenz is associated with severe Vitamin D deficiency and increased alkaline phosphatase. AIDS. 2010;24:1923-8.
- [Google Scholar]
- High prevalence of and progression to low bone mineral density in HIV-infected patients: A longitudinal cohort study. AIDS. 2010;24:2827-33.
- [Google Scholar]
- Bone mass and mineral metabolism in HIV+ postmenopausal women. Osteoporos Int. 2005;16:1345-52.
- [Google Scholar]
- Lean mass is the strongest predictor of bone mineral content in type-2 diabetes and normal individuals: An eastern India perspective. J Diabetes Metab Disord. 2014;13:90.
- [Google Scholar]
- CD4+ cell count, viral load, and highly active antiretroviral therapy use are independent predictors of body composition alterations in HIV-infected adults: A longitudinal study. Clin Infect Dis. 2005;41:1662-70.
- [Google Scholar]
- How best to approach endocrine evaluation in patients with HIV in the era of combined antiretroviral therapy? Clin Endocrinol (Oxf). 2013;79:310-3.
- [Google Scholar]
- Official positions of the International Society for Clinical Densitometry. J Clin Densitom. 2004;7:1-6.
- [Google Scholar]
- Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-23.
- [Google Scholar]
- HIV: An underrecognized secondary cause of osteoporosis? J Bone Miner Res. 2013;28:1256-8.
- [Google Scholar]
- HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem. 2003;278:48251-8.
- [Google Scholar]
- Prevalence of Vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: A community-based survey. Br J Nutr. 2009;102:382-6.
- [Google Scholar]
- Vitamin-D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: An open label randomized prospective study from Eastern India. Diabetes Res Clin Pract. 2014;103:e18-23.
- [Google Scholar]
- Reduced bone mineral density in human immunodeficiency virus-infected patients and its association with increased central adiposity and postload hyperglycemia. J Clin Endocrinol Metab. 2004;89:1200-6.
- [Google Scholar]
- Prevalence and risk factors of osteopenia/osteoporosis in Turkish HIV/AIDS patients. Braz J Infect Dis. 2013;17:707-11.
- [Google Scholar]
- Prevalence of low bone mineral density among HIV patients on long-term suppressive antiretroviral therapy in resource limited setting of Western India. J Int AIDS Soc. 2014;17(4 Suppl 3):19567.
- [Google Scholar]
- Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554-61.
- [Google Scholar]
- Prevalence of low bone mineral density in a low-income inner-city population. J Bone Miner Res. 2011;26:388-96.
- [Google Scholar]
- Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin Infect Dis. 2013;57:1483-8.
- [Google Scholar]
- The frequency of low muscle mass and its overlap with low bone mineral density and lipodystrophy in individuals with HIV – A pilot study using DXA total body composition analysis. J Clin Densitom. 2012;15:224-32.
- [Google Scholar]
- Increased abdominal visceral fat is associated with reduced bone density in HIV-infected men with lipodystrophy. AIDS. 2001;15:975-82.
- [Google Scholar]
- Relationship between body composition and bone mineral density (BMD) in perimenopausal Korean women. Clin Endocrinol (Oxf). 2009;71:18-26.
- [Google Scholar]
- Static vs. dynamic loads as an influence on bone remodelling. J Biomech. 1984;17:897-905.
- [Google Scholar]
- Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr. 2011;52:71-4.
- [Google Scholar]
- Effect of alendronate on HIV-associated osteoporosis: A randomized, double-blind, placebo-controlled, 96-week trial (ANRS 120) AIDS Res Hum Retroviruses. 2012;28:972-80.
- [Google Scholar]
- Effects of intravenous zoledronate on bone turnover and bone density persist for at least five years in HIV-infected men. J Clin Endocrinol Metab. 2012;97:1922-8.
- [Google Scholar]






