Translate this page into:
Circulation of Nipah virus in Pteropus giganteus bats in northeast region of India, 2015
*For correspondence: dtmourya@gmail.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Nipah virus (NiV) is a member of the family Paramyxoviridae, genus Henipavirus and an important emerging zoonotic disease in South East Asia1. NiV was first identified in Singapore and Malaysia during an outbreak of severe febrile illness with encephalitis in 1998-199923. In 2001, Nipah encephalitis outbreak was reported for the first time from Meherpur district, Bangladesh; since then, sporadic cases and outbreaks were reported from various districts of Bangladesh456. India has witnessed two outbreaks of Nipah encephalitis in the eastern State of West Bengal during the year 2001 and 200778.
The northeast region of India is bordered by Bangladesh, where sporadic cases and outbreaks of Nipah have been frequently reported. India represents an incredible diversity of bats with at least 117 species and 100 subspecies under 30 genera belonging to eight families. Around 62 species of bat are found to be widely distributed in different areas of northeastern States of India910. Pteropus bats are known to be a natural reservoir for NiV; however, the role of these bats in concern with outbreaks among human populations from Nadia and Siliguri district of West Bengal could not be associated. Further, no information is available for the presence of NiV from Assam State which shares a border with West Bengal11. In view of the above, the present study was conducted to understand the circulation of NiV in bats from the Assam and West Bengal States.
A survey was conducted in West Bengal and Assam States during three-field visits in March, May and December 2015. Various locations were selected based on roosting areas of Pteropus bats in West Bengal and Assam that share boundaries with Bangladesh. Prior permission for animal capture was obtained from the Chief Conservators of Forests of West Bengal and Assam. Institutional Animal Ethical Committee of the ICMR-National Institute of Virology (NIV), Pune, India, approved this study. All laboratory work was performed in NIV, Pune.
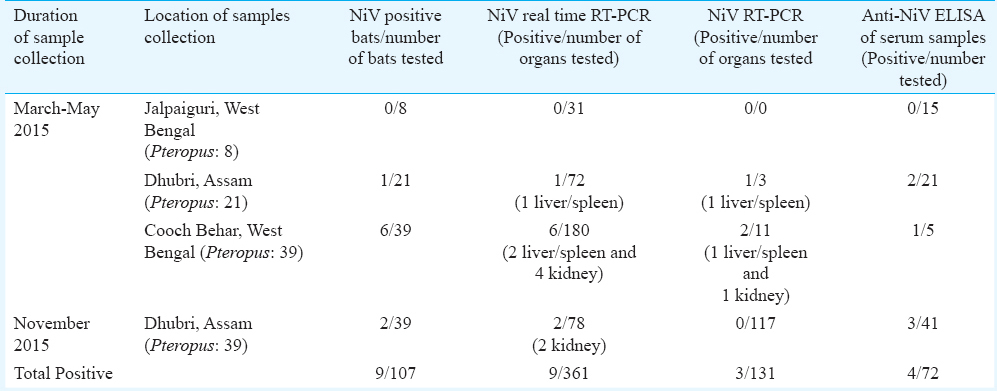
One hundred and seven Pteropus giganteus bats were captured from Jalpaiguri (n=8), Cooch Behar (n=39) districts of West Bengal and Dhubri (n=60) district of Assam State during three field visits (Figure). Bat necropsies were performed in the field following biosafety protocols using personal protective equipment, including powered air-purifying respirators. Blood, organs (kidney, liver and spleen), throat swabs, rectal swabs and urine samples were collected.
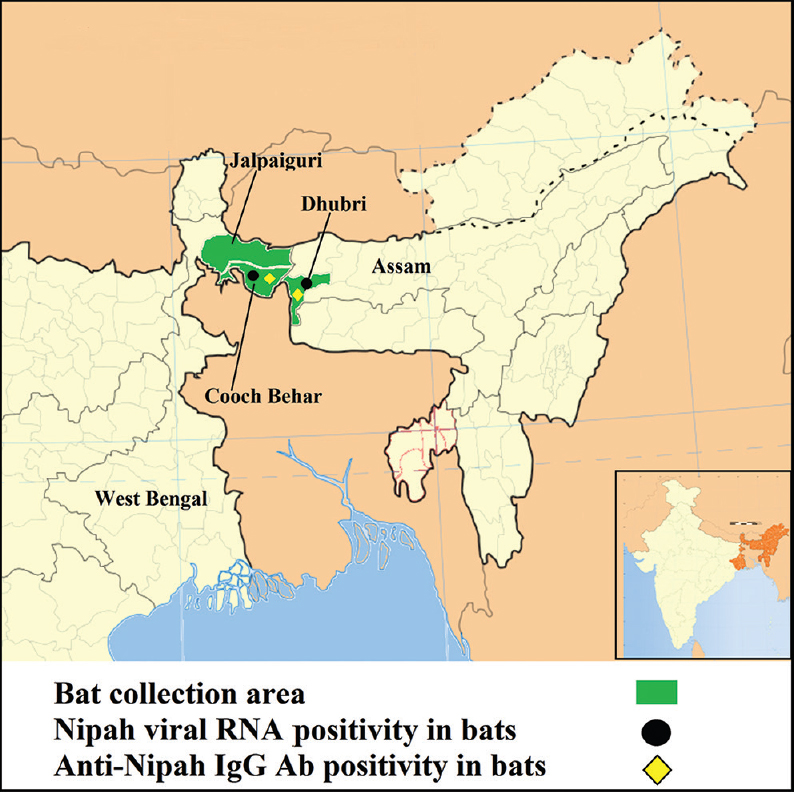
- Geographic locations of bat collection and for Nipah positivity in Pteropus giganteus bat, northeast India.
Of the 107 P. giganteus bat tissue specimens (liver/spleen and kidney) tested for NiV by real-time reverse transcriptase polymerase chain reaction (RT-PCR)12, nine bats (6 bats from Cooch Behar district of West Bengal and 3 from Dhubri district of Assam) were found to be positive for NiV RNA (Table). Viral RNA from tissue samples of only one bat from Cooch Behar district of West Bengal and one bat from Dhubri district of Assam could be amplified using partial nucleocapsid gene-specific reverse transcriptase nested PCR (100 bp) as described earlier78. This was further confirmed by DNA sequencing of partial nucleocapsid gene. BLAST results suggested the highest homology of 99 per cent with known sequences of P. giganteus-derived NiV nucleoprotein sequences from India (AFJ513078) and Bangladesh (AY988601). This indicated that NiV strain circulating in India and Bangladesh shared the highest homology; however, it was divergent from Malaysian strains8.
Virus isolation was attempted using in vivo and in vitro systems. Liver/spleen and kidney tissues of NiV-positive bats (n=9) were homogenized in sterile Minimum Essential Medium (MEM; Gibco, USA) using a homogenizer (GenoGrinder 2000; BT&C Inc., Lebanon, NJ, USA). Further, tissue homogenates were centrifuged at 1984 g for 10 min, and 0.1 ml of the supernatants was applied to monolayers of Vero CCL-81 cells grown in 24-well cell culture plates after removing the growth medium. The cells were incubated for 1 h at 37°C to allow virus adsorption, with rocking every 10 min for uniform distribution of inoculum. After the incubation, the cells were washed with phosphate buffer saline (1×, pH 7.4), and finally, MEM supplemented with 2 per cent foetal bovine serum was added to each well. The cultures were incubated further in 5 per cent CO2 incubator at 37°C and observed daily for cytopathic effects under an inverted microscope11.
NiV-positive bat (n=4) tissue specimens were also used for virus isolation in Hamster model13. No clinical signs were observed in experimentally infected hamsters up to 30 days post-inoculation. Hamsters were euthanized and blood, organs were harvested. The organs (liver/spleen, kidney and brain) and serum samples were tested for NiV by real-time PCR. All the samples were found to be negative for NiV RNA. Three blind passages were made using NiV RNA-positive bat tissue samples in both in vitro and in vivo (Hamster model) system, but virus isolation could not be obtained.
Available P. giganteus bat serum samples (n=71) were tested using anti-NiV IgG ELISA. Of these, four were positive for anti-NiV IgG antibodies (antibody titer 1:100) (3 from Dhubri district, Assam and 1 from Cooch Behar district, West Bengal State) (Table). Two bats were found to be positive for both, viral RNA and IgG antibodies. Throat swabs, rectal swabs and urine samples were found to be negative by NiV-specific real-time RT-PCR12.
The above findings indicated the presence of NiV among Pteropus bats from West Bengal and its new niche in Assam State. During our earlier studies, we have documented the presence of NiV and associated encephalitis outbreak in West Bengal State711. During the present study, it was observed that large colonies/roosts of P. giganteus bats were present in close proximity of human settlements in Dhubri, Assam, and Cooch Behar district of West Bengal State. This signifies higher and easier chances of virus spillover from bats to human population1. The presence of NiV in the bat population in a previously unexplored region is a matter of serious concern. This warrants implementation of necessary steps for a survey to determine the presence of NiV among human, suspected cases and reservoirs (swine and bat) in other States of northeast India.
The limitation of the study was the failure to amplify and sequence other regions of NiV genome using the available clinical material. This would have given more insight about the current NiV strain detected in Assam.
Acknowledgment
Authors acknowledge the support extended by the Secretary, DHR, Government of India and Director General, Indian Council of Medical Research, New Delhi, and thank the Principal Conservators of Forests of Assam and West Bengal and other concerned officials for their permission to capture bats. Authors are thankful to Dr M.S. Chadha, Group leader, Influenza department for continuous guidance and support and Dr. R Laxminarayanan, Senior Administrative Officer, NIV, Pune, for providing logistic support. Technical assistance rendered by S.V. Gopale, M. Holeppanavar, M. Acharya, M. Karnjavane, G.K. Chopade, S. Dhaigude, D.K. Singh (field) and D. Bhattad, S. Melag and U.K. Shende (laboratory) is gratefully acknowledged.
Financial support & sponsorship: The authors acknowledge the ICMR for funding extramural project ID: 2013:1445 ‘Multi-site epidemiological and virological survey of NiV: special emphasis on Northeast region of India’ under Northeast task force programme
Conflicts of Interest: None.
References
- Emerging infectious diseases in Southeast Asia: Regional challenges to control. Lancet. 2011;377:599-609.
- [Google Scholar]
- Fatal encephalitis due to Nipah virus among pig-farmers in Malaysia. Lancet. 1999;354:1257-9.
- [Google Scholar]
- Nipah virus encephalitis reemergence, Bangladesh. Emerg Infect Dis. 2004;10:2082-7.
- [Google Scholar]
- Pathogenic differences between Nipah virus Bangladesh and Malaysia strains in primates: Implications for antibody therapy. Sci Rep. 2016;6:30916.
- [Google Scholar]
- Nipah virus transmission from bats to humans associated with drinking traditional liquor made from date palm sap, Bangladesh, 2011-2014. Emerg Infect Dis. 2016;22:664-70.
- [Google Scholar]
- Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006;12:235-40.
- [Google Scholar]
- Genomic characterization of Nipah virus, West Bengal, India. Emerg Infect Dis. 2011;17:907-9.
- [Google Scholar]
- Order Chiroptera. In: Wilson DE, Reeder DM, eds. Mammal Species of the World: A Taxonomic and Geographic Reference Vol 1. (3rd ed). Baltimore: Johns Hopkins University Press; 2005. p. :312-529.
- [Google Scholar]
- Contribution to the knowledge of Bats (Mammalia: Chiroptera) of North East Hills India. In: Records of the Zoological Survey of India. Occassional Paper No. 174. Kolkata: Zoological Survey of India; 1999.
- [Google Scholar]
- Detection of Nipah virus RNA in fruit bat (Pteropus giganteus) from India. Am J Trop Med Hyg. 2012;87:576-8.
- [Google Scholar]
- Specific detection of Nipah virus using real-time RT-PCR (TaqMan) J Virol Methods. 2004;120:229-37.
- [Google Scholar]
- A golden hamster model for human acute Nipah virus infection. Am J Pathol. 2003;163:2127-37.
- [Google Scholar]





