Translate this page into:
Assessment of potential biomarkers of atherosclerosis in Indian patients with type 2 diabetes mellitus
For correspondence: Dr. Sudha S. Deo, Sir HN Medical Research Society, Sir HN Reliance Foundation Hospital & Research Centre, Rajaram Mohan Roy Road, Prarthana Samaj, Girgaum, Mumbai 400 004, Maharashtra, India e-mail: sudha.deo@hnhospital.com
-
Received: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Various biological markers of subclinical atherosclerosis have been proposed to predict cardiovascular events in patients with diabetes mellitus (DM). However, there are only a few clinical studies assessing the role of invasive biomarkers [CD-36, peroxisome proliferator-activated receptor gamma (PPAR-γ) and YKL-40] in Indian patients with type 2 DM (T2DM). Hence, the present study was conducted to assess protein levels and gene expression of CD-36, PPAR-γ and YKL-40 in patients with T2DM and compare that with hypertensive and healthy controls.
Methods:
All the participants were subjected to medical history, anthropometric measurements and biochemical and biomarker (ELISA and real-time polymerase chain reaction) estimations. The study groups consisted of patients with T2DM (>5 yr) with hypertension (n=55), patients with T2DM (<2 yr) without hypertension (n=28), hypertensive controls (n=31) and healthy controls (n=30).
Results:
Gene expressions of YKL-40 and CD36 were significantly higher in patients with T2DM (>5 yr) with hypertension compared to healthy controls (P=0.006). In addition, a significant increase in serum levels of sCD36, PPAR-γ and YKL-40 was observed in patients with T2DM (>5 yr) with hypertension compared to healthy controls (P< 0.05). Serum levels as well as gene expression of CD36 showed significant correlation with serum levels as well as gene expression of PPAR-γ (ρ=0.45 and ρ=0.51; P< 0.001), respectively.
Interpretation & conclusions:
CD36 and YKL-40 may be potential inflammatory biomarkers for early onset of atherosclerosis in patients with T2DM.
Keywords
CD36
peroxisome proliferator-activated receptor gamma
type 2 diabetes mellitus
YKL40
Diabetes mellitus (DM) is a worldwide health concern affecting all the ages. It is on the edge of becoming a pandemic in India, with more than 72 million reported cases in the year 20171. Autoimmune destruction of β-cells results in type 1 DM (T1DM), whereas conditions that reduce insulin sensitivity and adversely affect β-cell activities result in T2DM2.
DM is strongly associated with both microvascular (retinopathy, nephropathy and neuropathy) and macrovascular [coronary artery disease (CAD), peripheral arterial disease and stroke] complications, resulting in organ and tissue damage in approximately one-third to one-half of patients3. Atherosclerosis is the central pathological mechanism in macrovascular disease leading to narrowing of arterial walls. There is evidence supporting the central role of endothelium and inflammation in all phases of the atherosclerotic process4. Patients with T2DM have insulin resistance that results in hyperglycaemia, dyslipidaemia, hypertension and clotting abnormalities, which in turn act as inciting stimuli for early onset of atherosclerosis5.
Potential biomarkers such as CD36, peroxisome proliferator-activated receptor gamma (PPAR-γ) and YKL-40 may play significant roles in insulin resistance and atherosclerosis in patients with T2DM. CD36 is a multi-ligand scavenger receptor present on the surface of monocyte/macrophages. It binds and endocytoses oxidized low-density lipoprotein (LDL) and is implicated in the formation of foam cells. Thus, CD36 plays a critical role in the development of atherosclerotic lesions6. Expression of scavenger receptor CD36 is increased in the presence of PPAR-γ7. It is well documented that patients with CAD express significantly higher levels of both PPAR-γ protein (approximately 10-fold) and mRNA (approximately 60-fold) compared with healthy volunteers8. YKL-40 is a novel biomarker expressed and secreted by macrophages. YKL-40 mRNA expression is highly upregulated on macrophages, specifically those that infiltrate deeper into the atherosclerotic lesion9.
Various biological markers have been proposed to act as predictors of cardiovascular events in patients with DM. It is important to have a detailed understanding of these biomarkers to clarify the biological action of cytokines and endothelial dysfunction in the occurrence of insulin resistance. Hence we undertook this study to evaluate the role of these potential biomarkers of subclinical atherosclerosis in patients with T2DM with or without hypertension and compare with hypertensive controls and healthy controls.
Material & Methods
The study consisted of patients with T2DM for more than five years along with hypertension (Group A-I, n=55), newly diagnosed patients with T2DM without hypertension (Group A-II, n=28), patients with essential hypertension only (hypertensive controls; Group B-I, n=31) and healthy controls (Group B-II, n=30). All patients were recruited consecutively over a period of a year and five months (November 2011 to April 2013) from the Diabetes Clinic of Sir HN Hospital, Mumbai, India. Patients with significant systemic disease (except DM and hypertension for Groups A-I, A-II and B-I) including autoimmune or chronic inflammatory conditions, patients on thiazolidinediones (PPAR-γ agonist) and direct vasodilators and pregnant and lactating women were excluded. Healthy controls were adjudged healthy based on medical history, physical examination and laboratory investigations. All participants were above 40 yr of age and of either gender, who were recruited from the hospital staff.
This was a hypothesis-generating study of assessing the role of multiple biomarkers and their genes in Indian population. Hence, no formal sample size calculation was done. However, based on the available literature on these genes, the sample size of 30 per group was deemed appropriate to get significant association between T2DM and various biomarkers. It was decided to enrol participants in 2:1:1:1 ratio for this study.
The study was approved by the Institutional Ethics Committee. A written informed consent was obtained from all the participants.
Biochemical examinations: Demographic details, personal as well as family medical history, with a history of smoking, alcohol or substance abuse, were recorded. Anthropometric measurements such as height (in centimetre), weight (in kilogram), body mass index (BMI) and waist-to-hip ratio were measured. Two readings of blood pressure (systolic and diastolic) were recorded using mercury sphygmomanometer and the average was used for analysis.
All the investigations were performed after overnight fasting. Five millilitres of blood was collected from each participant in ethylenediaminetetraacetic acid (EDTA) and plain bulb. After centrifugation, serum sample was used for estimation of fasting blood sugar and lipid profile (Konelab® prime 30 automatic analyzer, Thermo Fisher Scientific, USA). EDTA sample was used for analyzing glycosylated haemoglobin. Postprandial blood sugar was measured at two hours post-lunch. All the laboratory analyses were done within two hours of sample collection.
Protein estimations: Serum YKL-40, PPAR-γ and CD36 (soluble) were analyzed with a commercial assay on the serum sample stored at −80°C. YKL-40 was analyzed by Human Chitinase 3-like 1 Quantikine ELISA Kit by R&D Systems, 614 McKinley Pl NE, Minneapolis, USA [range, 15.9-93.5 ng/ml; coefficient of variation (CV) <5%]. PPAR-γ was analyzed by Human Peroxisome Proliferator-activated receptor γ (PPAR-γ) ELISA Kit by MyBioSource, San Diego, USA (range, 2-600 ng/ml; CV <10%) and CD36 was analyzed by Human Soluble Cluster of Differentiation 36 (sCD36) ELISA kit by MyBioSource, San Diego, USA (range, 2-110 ng/ml; CV <10%).
Gene expressions: Gene expressions of all the biomarkers, CD36, PPAR-γ and YKL-40, were studied on monocytes. A method developed by Graziani-Bowering et al10 was used to separate monocytes from lymphocytes on the basis of rate of flotation from leucocyte-rich plasma. This method provided highly purified and viable monocytes. Cytoplasmic RNA (NucleoSpin, Macherey-Nagel, Germany) was prepared and the first-strand cDNA was synthesized (High Capacity® Reverse Transcription kit, Applied Biosystems, USA) according to the manufacturer's instructions.
Real-time polymerase chain reaction was performed with the Step One plus system from Applied Biosystems (Foster City, California, USA), using TaqMan® Gene Expression Assay (Foster City, California, USA) and specific primers and probes for PPAR-γ, CD36, YKL-40 and β-Actin. Relative gene expression was calculated by the 2−ΔΔct method11. Data are shown as the fold change in expression of the target gene relative to the internal control gene (β-Actin).
Statistical analysis: Numerical data were tested for normality using Kolmogorov-Smirnov test, and between groups comparison was done using either one-way analysis of variance and post hoc unpaired t test (if normally distributed) or Kruskal-Wallis test and post hoc Mann-Whitney U-test (if not normally distributed). Categorical data were compared using Chi-square test. Correlation between two numerical variables (as they were not normally distributed) was assessed using Spearman's rho correlation coefficient. All analyses were performed using SPSS software, version 21.0 (SPSS, Chicago, IL, USA).
Results
A total of 144 participants were enrolled in this study. Table I shows that there were no significant differences in the distribution of demographic details and anthropometric measurements, except for blood pressure, across the four study groups. Systolic blood pressure was significantly elevated in patients with T2DM (>5 yr) with hypertension as compared to patients with T2DM (<2 yr) without hypertension (P=0.02) and healthy controls (P=0.006). Diastolic blood pressure was significantly higher in patients with T2DM (>5 yr) with hypertension as compared to patients with T2DM (<2 yr) without hypertension (P=0.01).
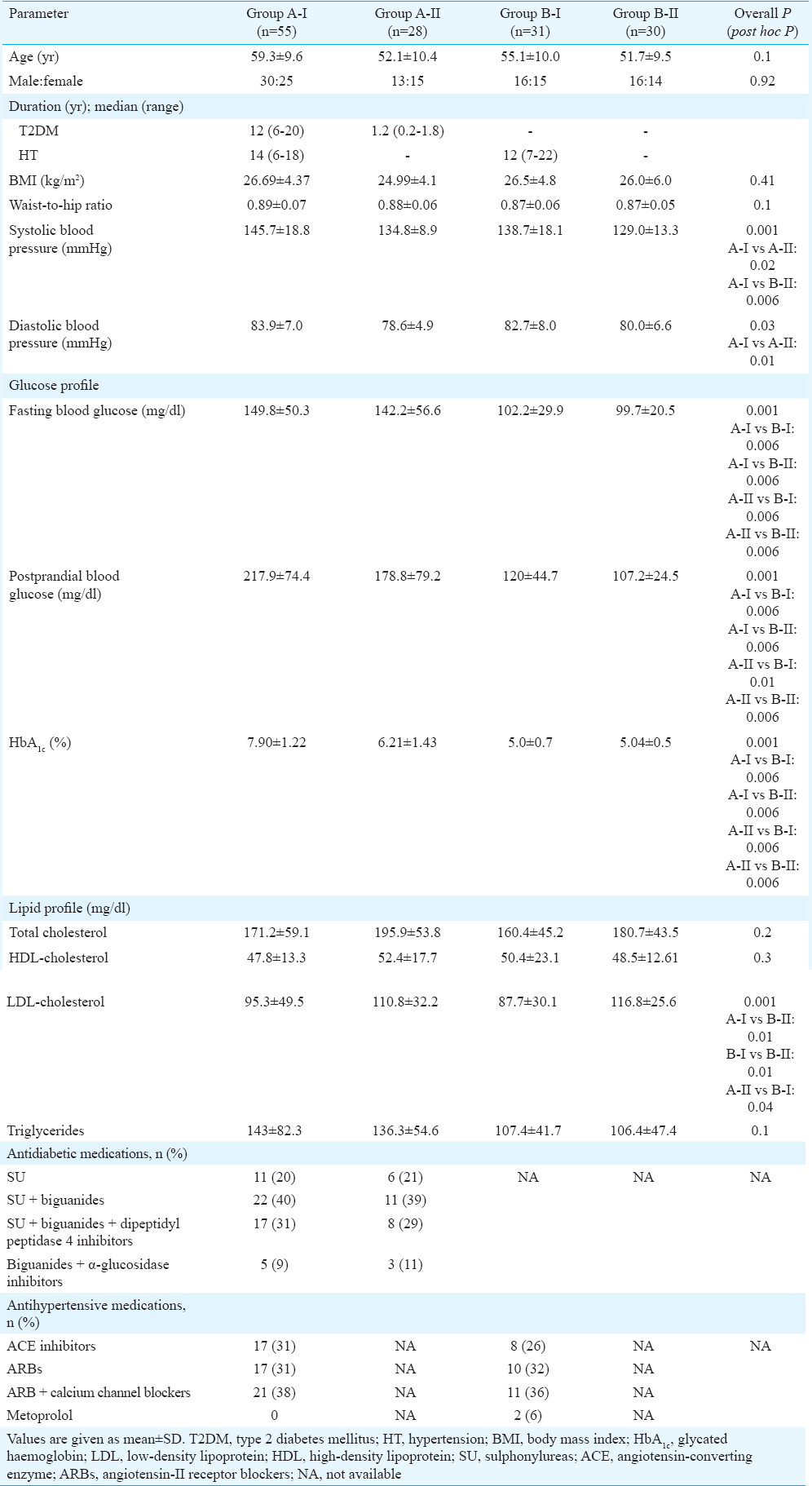
Patients with hypertension were treated with one or more of the following classes of medications - angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists, calcium channel blockers and cardioselective beta-blockers. Patients with T2DM were on either monotherapy or combination therapy consisting of the following classes of antidiabetic medications – biguanides, sulphonylureas, dipeptidyl peptidase IV inhibitors and alpha-glucosidase inhibitors. Biochemical parameters such as blood glucose (fasting and postprandial) and glycated haemoglobin (HbA1c) were significantly higher in patients with T2DM compared to hypertensive controls and healthy controls (both P=0.006). A significant reduction was observed in serum LDL-cholesterol in patients with T2DM (>5 yr) with hypertension and hypertensive controls compared to healthy controls.
Serum biomarkers: Serum levels of soluble CD36 (sCD36), YKL-40 and PPAR-γ were significantly (P < 0.001) elevated in patients with T2DM (>5 yr) with hypertension when compared to hypertensive and healthy controls. Serum levels of sCD36, YKL-40 and PPAR-γ were significantly elevated in patients with T2DM (<2 yr) without hypertension as compared to healthy controls (Table II).
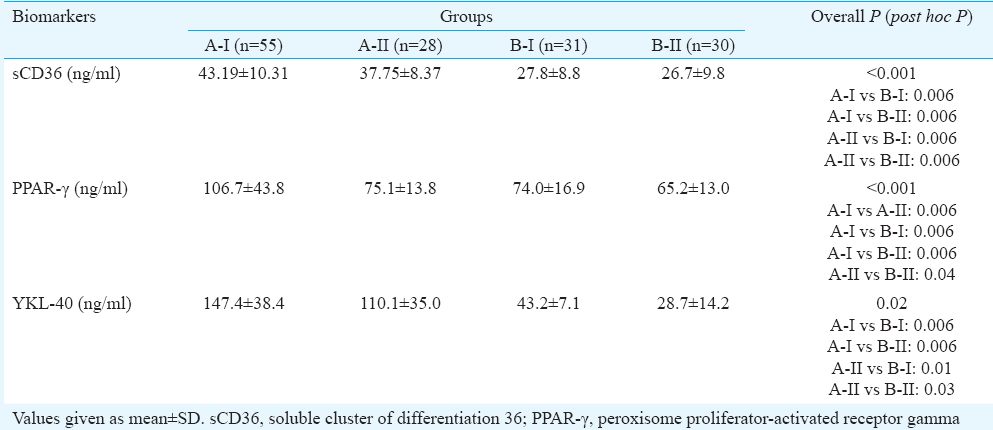
Gene expression of biomarkers: Expression of YKL-40 and CD36 genes was significantly higher in patients with T2DM (>5 yr) with hypertension as compared to healthy controls (Table III).
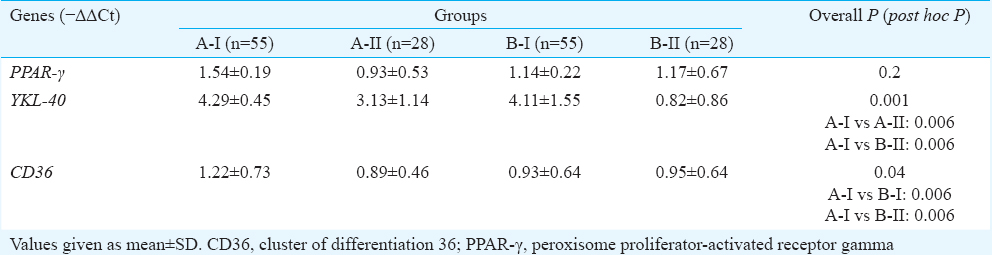
Correlation studies: Significant correlation was observed between the expression of CD36 and PPAR-γ genes (ρ=0.51, P< 0.001). Serum levels of CD36 also showed significant correlation with serum levels of PPAR-γ (ρ=0.45, P<0.001).
A significant correlation was observed between serum levels and gene expression of YKL-40 (ρ=0.48, P< 0.001) as represented in Figure. However, there was no significant correlation between serum levels and gene expression of either CD36 or PPAR-γ. There was no significant correlation between HbA1c and serum levels of either CD36 or YKL-40 or PPAR-γ.
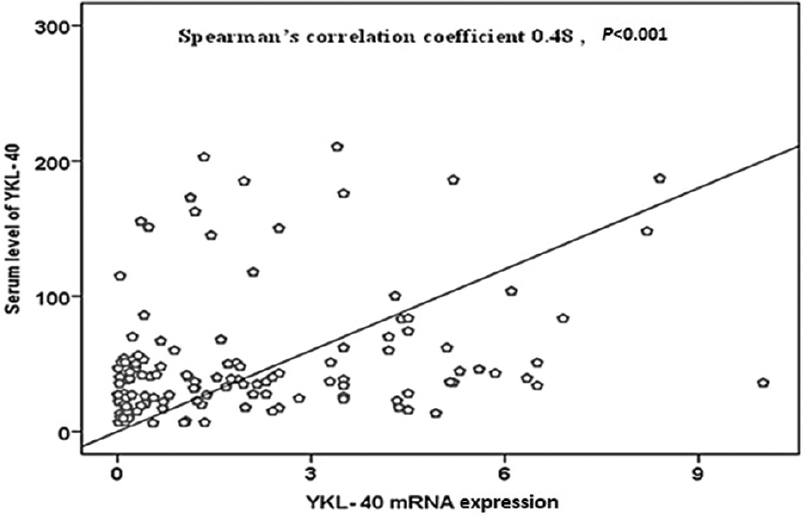
- Correlation between serum levels and gene expressions of YKL-40.
Discussion
The results of the present study showed that the serum protein levels of CD36, YKL-40 and PPAR-γ were significantly higher in patients with T2DM compared to healthy controls. The gene expression of YKL-40 and CD36 was significantly higher in patients with T2DM (>5 yr) as compared to healthy controls. Serum levels and gene expression of CD36 showed significant correlation with serum levels and gene expression of PPAR-γ, respectively. A significant, moderate correlation was observed between serum levels and gene expression of YKL-40.
CD36 binds and internalizes modified LDL, which facilitates the formation of lipid-engorged macrophage foam cells. It is believed to play a vital role in the initiation and progression of atherosclerosis12. Thus, CD36 has been implicated in conditions related with metabolic deregulation, which includes obesity, insulin resistance, DM, diabetic nephropathy and atherosclerosis13. This may explain the accelerated CD36 expression in parallel with the progression of atherosclerosis.
Our study showed increased serum sCD36 levels in patients with T2DM as compared to healthy controls as substantiated by Handberg et al14 and Alkhatatbeh et al15. CD36 mRNA expression on monocytes was significantly higher in patients with T2DM (>5 yr) as compared to hypertensive and healthy controls. This was in accordance with Han et al16 who reported that CD36 mRNA expression was parallel to the increase in CD36 protein levels in serum.
Studies have documented that CD36 expression on monocytes is upregulated by ox-LDL whose levels increase in case of T2DM and related atherosclerosis. sCD36 is also a marker of plaque instability and symptomatic carotid atherosclerosis, possibly as a result of CD36 release to the circulation from the foam cells within the atherosclerotic lesion17. Another study has shown an increase in CD36 transcript in the presence of elevated glucose; this provides a mechanism for understanding accelerated atherosclerosis in patients with DM18. This suggests that patients with T2DM with elevated sCD36 might show early onset of subclinical atherosclerosis.
PPAR-γ ligands have an impact on all vascular cells relevant to the development of atherosclerosis: vascular smooth muscle cells, endothelial cells (ECs) and monocyte/macrophages19. PPAR-γ regulates a variety of cellular processes that have an effect on glucose homeostasis, endothelial function and vessel wall inflammation. It also increases the expression of CD36. Our findings were in accordance with this function of PPAR-γ. PPAR-γ agonists are useful for patients with T2DM because they decrease hepatic glucose production and prolong pancreatic β-cell function by preventing apoptosis of β-cells20. The serum levels of PPAR-γ were found to be significantly higher in patients with T2DM (>5 yr) than other study groups. In the present study, there was no significant difference in gene expression of PPAR-γ across the study groups. These findings were in agreement with those of Teupser et al21.
YKL-40 is involved in endothelial dysfunction in patients with T2DM22. In vitro studies also show that YKL-40 promotes chemotaxis, cell attachment and spreading and migration of vascular ECs, which suggest a role of YKL-40 in the atherosclerotic plaque formation22. High YKL-40 mRNA expression was seen in macrophages that infiltrate deeper in the atherosclerotic lesion and the highest expression of YKL-40 protein was seen in macrophages in the early lesion of atherosclerosis23.
In the present study, serum levels as well as gene expression of YKL-40 were significantly increased in patients with T2DM (<5 yr with hypertension) as compared to healthy controls. Nielsen et al24 found elevated plasma levels of YKL-40 in patients with T2DM compared to healthy controls. It was proposed that YKL-40 might be involved in glucose metabolism.
Michelsen et al25 have found that YKL-40 might be a marker of plaque instability, potentially reflecting macrophage activation and matrix degradation inside the atherosclerotic lesion. Kastrup et al26 have demonstrated that circulating YKL-40 may reflect total burden of coronary atherosclerosis or may help to identify high-risk atherosclerosis phenotype. Røndbjerg et al27 also suggested a role of YKL-40 in the progressing vascular complications in patients with T2DM. Elevated YKL-40 levels have also been found to be associated with all-cause as well as cardiovascular mortality in patients with stable CAD. Thus, elevated YKL-40 in patients with long-term T2DM might help to identify early atherosclerosis in these patients.
A significant correlation between serum levels and gene expression was observed only for YKL-40, but not for CD36 or PPAR-γ. The difference between gene expression and serum levels may be due to non-monocyte gene expression, differential secretion from monocytes in different patient groups and differences in clearance of the serum biomarkers.
The present study was limited by the cross-sectional design and no long-term follow up of these patients to assess the risk of developing cardiovascular events in different study groups. The parts of the present study on non-invasive biomarkers28 and on the effect of small dense (sd) LDL-C on cardiovascular risk in patients with T2DM29 have already been published.
Inflammatory biomarkers such as CD36 and YKL-40 play significant roles in atherogenic processes, including foam cell formation, plaque instability and release of inflammatory mediators. Assessment of these biomarkers in patients with T2DM might aid in understanding the pathogenic mechanisms leading to cardiovascular diseases. Future research should focus on validating CD36 and YKL-40 in diverse and large population with T2DM including those with insulin resistance.
Financial support & sponsorship: None
Conflicts of Interest: None.
References
- International Diabetes Federation. IDF SEA Region. Available from: https://www.idf.org/our-network/regions-members/south-east-asia/members/94-india.html
- [Google Scholar]
- Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol. 2013;9:513-21.
- [Google Scholar]
- UK Prospective Diabetes Study (UKPDS). VIII. Study design, progress and performance. Diabetologia. 1991;34:877-90.
- [Google Scholar]
- Endothelial function and inflammation in coronary artery disease. Heart. 2006;92:441-4.
- [Google Scholar]
- Molecular insights and therapeutic targets for diabetic endothelial dysfunction. Circulation. 2009;120:1266-86.
- [Google Scholar]
- Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678-89.
- [Google Scholar]
- YKL-40, a biomarker of inflammation, is elevated in patients with type 2 diabetes and is related to insulin resistance. Inflamm Res. 2006;55:53-9.
- [Google Scholar]
- A quick, easy and inexpensive method for the isolation of human peripheral blood monocytes. J Immunol Methods. 1997;207:157-68.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-8.
- [Google Scholar]
- Analysis of gene and protein expression during monocyte-macrophage differentiation and cholesterol loading – CDNA and protein array study. Atherosclerosis. 2005;180:283-91.
- [Google Scholar]
- CD36: A class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785-91.
- [Google Scholar]
- Soluble CD36 in plasma is increased in patients with symptomatic atherosclerotic carotid plaques and is related to plaque instability. Stroke. 2008;39:3092-5.
- [Google Scholar]
- Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor, CD36. J Biol Chem. 1997;272:21654-9.
- [Google Scholar]
- A link between diabetes and atherosclerosis: Glucose regulates expression of CD36 at the level of translation. Nat Med. 2001;7:840-6.
- [Google Scholar]
- Troglitazone inhibits vascular smooth muscle cell growth and intimal hyperplasia. J Clin Invest. 1996;98:1897-905.
- [Google Scholar]
- Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:176-85.
- [Google Scholar]
- Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci U S A. 1999;96:11513-8.
- [Google Scholar]
- CD36 mRNA expression is increased in CD14+ monocytes of patients with coronary heart disease. Clin Exp Pharmacol Physiol. 2008;35:552-6.
- [Google Scholar]
- Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 1999;250:168-73.
- [Google Scholar]
- Strong induction of members of the chitinase family of proteins in atherosclerosis: Chitotriosidase and human cartilage gp-39 expressed in lesion macrophages. Arterioscler Thromb Vasc Biol. 1999;19:687-94.
- [Google Scholar]
- Plasma YKL-40: A BMI-independent marker of type 2 diabetes. Diabetes. 2008;57:3078-82.
- [Google Scholar]
- Increased YKL-40 expression in patients with carotid atherosclerosis. Atherosclerosis. 2010;211:589-95.
- [Google Scholar]
- High serum YKL-40 concentration is associated with cardiovascular and all-cause mortality in patients with stable coronary artery disease. Eur Heart J. 2009;30:1066-72.
- [Google Scholar]
- YKL-40 levels are independently associated with albuminuria in type 2 diabetes. Cardiovasc Diabetol. 2011;10:54.
- [Google Scholar]
- Effect of age and blood pressure on surrogate markers of atherosclerosis in patients with type 2 diabetes mellitus. J Clin Diagn Res. 2014;8:BC08-11.
- [Google Scholar]
- Assessment of small, dense low density lipoprotein cholesterol as a marker of cardiovascular risk in Indian patients with type 2 diabetes mellitus. IJAR. 2015;5:585-90.
- [Google Scholar]






