Translate this page into:
Speciation, clinical profile & antibiotic resistance in Aeromonas species isolated from cholera-like illnesses in a tertiary care hospital in north India
Reprint requests: Dr. Neelam Taneja, Department of Medical Microbiology, Research Block A, Postgraduate Institute of Medical Education & Research, Sector 12, Chandigarh 160 012, India e-mail: drneelampgi@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Aeromonas species have been reported to cause various illnesses in humans such as wound infections, septicaemia, peritonitis and pneumonia. Their role in causation of cholera-like illness is also being increasingly recognized. This retrospective study was done to know the presence of Aeromonas as a cause of acute diarrhoea in a tertiary care hospital and to find the common species of Aeromonas causing diarrhoea and their antibiotic susceptibility patterns.
Methods:
Fifty isolates of Aeromonas were obtained over a period of 15 yr from 2000 to 2014 from patients of suspected acute gastroenteritis resembling cholera. Biotyping was done for 35 of these isolates available in culture collection, based on a panel of 13 biochemical reactions. Antibiogram was put up for all of these isolates by disk diffusion methods and interpreted according to the Clinical and Laboratory Standards Institute guidelines.
Results:
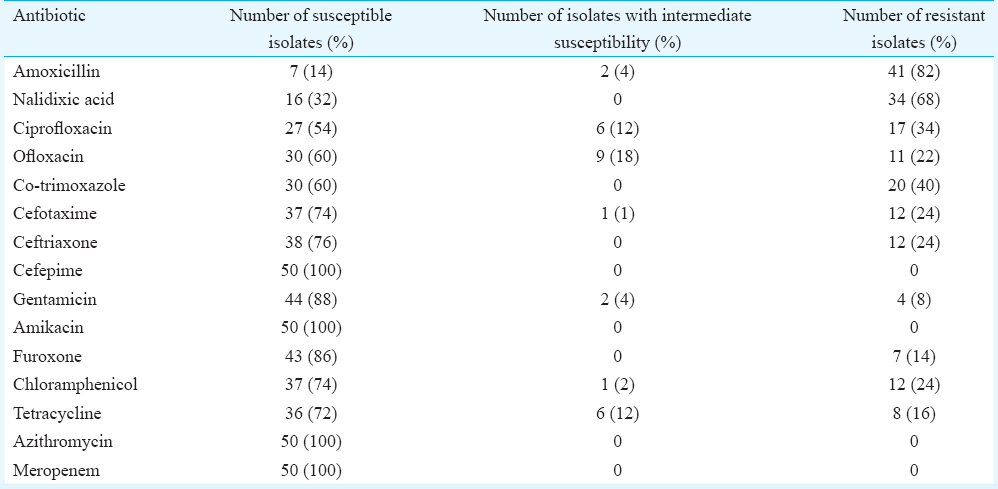
Of the 50 patients of Aeromonas-related acute gastroenteritis, 13 (26%) had typical features of cholera with rice water stools and severe dehydration. Eight patients (16%) had dysentery-like picture. One patient died of severe dehydration and septicaemia. The most common species were found to be Aeromonas caviae (34%) followed by Aeromonas veronii biovar veronii (29%), Aeromonas veronii biovar sobria (26%) and Aeromonas hydrophila (9%). All tested isolates were uniformly susceptible to cefepime, amikacin, azithromycin and meropenem; 14 per cent were susceptible to amoxicillin, 32 per cent to nalidixic acid, 60 per cent to co-trimoxazole, 54 per cent to ciprofloxacin, 60 per cent to ofloxacin, 74 per cent to chloramphenicol, 76 per cent to ceftriaxone, 74 per cent to cefotaxime, 88 per cent to gentamicin and 86 per cent to furoxone.
Interpretation & conclusions:
Aeromonas is an important, often neglected pathogen capable of causing a variety of gastrointestinal tract symptoms such as acute diarrhoea and dysentery and may even mimic cholera. It is, therefore, pertinent to recognize this pathogen as an important agent in the causation of severe diarrhoea.
Keywords
Acute gastroenteritis
Aeromonas
cholera-like illness
Diarrhoea is an important cause of morbidity and mortality, especially in the developing world. It is estimated that each year, 1.67 billion episodes of diarrhoea occur in children under five years of age and 430 million episodes occur in adults >16 yr of age, the burden being heaviest in Asian and African countries1. The pathogens causing diarrhoea are varied and numerous. According to the Global Enteric Multicenter Study (GEMS) study, the largest contributors to childhood diarrhoea are Rotavirus, Cryptosporidium, enterotoxigenic Escherichia coli and Shigella, other common agents being adenovirus 40/41, Aeromonas and Campylobacter2. Aeromonas was among the top five enteropathogens causing childhood diarrhoea in Asia, especially in Pakistan and Bangladesh2. Of the more dangerous forms of diarrhoea is cholera, caused by Vibrio cholerae, which leads to severe dehydration and high mortality if left untreated. Globally, it leads to about 120,000 deaths annually3. It is highly endemic in Southern Asia and India where it causes seasonal outbreaks as well as sporadic cases4.
Aeromonas, on the other hand, is a less well- characterized pathogen. It is a Gram-negative rod, facultatively anaerobic and is oxidase positive5. It normally inhabits aquatic environments, not only fresh water but also salty estuarine waters, causing soft tissue infections in aquatic animals. It is responsible mainly for three clinical syndromes i.e. septicaemia, wound infections and acute gastroenteritis. The acquisition of this infection can usually be traced back to ingestion of seafood or exposure to seawater or estuarine environment, in which the organism is seen to be present often causing disease in aquatic animals6. We describe here the results of a retrospective descriptive study of a period of 15 yr i.e. 2000-2014, of the cases of acute gastroenteritis and acute watery diarrhoea resembling cholera, due to Aeromonas, at a tertiary care hospital in north India.
Material & Methods
Diarrhoeal stool samples from 1595 patients suspected to have cholera were collected over a period of 15 yr between 2000 and 2014, irrespective of age group from both inpatient and outpatient facilities at Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. The samples were transported to the Enteric Microbiology Laboratory in Cary-Blair medium or in sterile containers and processed within two hours of receipt of samples in the laboratory. Patients’ clinicodemographic details were noted wherever available. These included age, sex, ward/outpatient department, presenting clinical features and underlying illness.
Bacteriological isolation: Conventional culture technique for isolation of stool pathogens was followed. In addition, samples were also inoculated onto 5 per cent sheep blood agar plate without ampicillin. Apart from direct plating, enrichment broths such as selenite-F and alkaline peptone water were used. Colonies showing typical characters of Aeromonas with haemolysis and oxidase positivity were sub-cultured onto 5 per cent sheep blood agar for further characterization. Species identification was done based on conventional biotyping according to the Aerokey II system with slight modifications following a panel of 13 biochemical reactions78. Isolates were stored in brain–heart infusion broth with 10 per cent glycerol at −80°C.
Antimicrobial susceptibility testing: Antimicrobial susceptibility testing of the Aeromonas isolates was carried out by the disk diffusion method of Kirby– Bauer9. Mueller-Hinton agar was used for susceptibility testing. Commercially available disks (HiMedia Laboratories Pvt. Ltd., Mumbai) of amikacin (30 µg), amoxicillin (10 µg), azithromycin (15 µg), cefepime (30 µg), cefotaxime (30 µg), ceftriaxone (30 µg), chloramphenicol (30 µg), ciprofloxacin (5 µg), co-trimoxazole (25 µg), furoxone (30 µg), gentamicin (10 µg), meropenem (10 µg), nalidixic acid (30 µg), ofloxacin (5 µg) and tetracycline (30 µg) were used. Standard strain of E. coli ATCC 25922 susceptible to all the drugs was used for quality control of disks. Results of zone diameters were interpreted as sensitive, intermediate resistant or resistant according to the Clinical and Laboratory Standards Institute criteria10.
Results
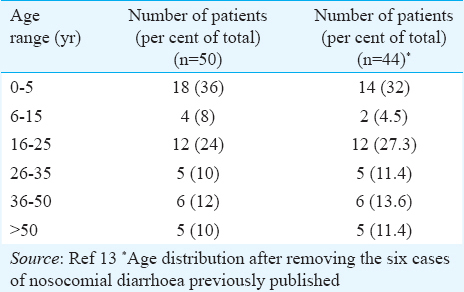
Aeromonas was isolated from 50 of the 1595 samples from patients suspected of cholera from 2000 to 2014. Age range of cases is depicted in Table I. Maximum number of patients (n=18) were seem during the months of July and August. Males outnumbered females by a ratio of 2:1. Of the 50 patients, 13 (26%) had severe dehydration and rice water stools typical of cholera. Eight patients (16%) had features of dysentery with bloody stools. One patient, from whom Aeromonas caviae was isolated, died after developing severe dehydration following consumption of seafood and alcohol at a party. Two patients had dual bacterial infections with Shigella sonnei and Salmonella Senftenberg, respectively. All others were monoinfections.
Of the 35 strains for which biochemical speciation was done, the most common species was found to be A. caviae (34%), followed by Aeromonas veronii biovar veronii (29%), Aeromonas veronii biovar sobria (26%) and Aeromonas hydrophila (9%). A single isolate of Aeromonas jandaei was obtained.
Antibiotic susceptibility testing was done for all 50 isolates (Table II). All tested isolates were uniformly susceptible to cefepime, amikacin, azithromycin and meropenem. Multidrug resistance was defined as non-susceptibility to ≥1 drug belonging to ≥3 classes of antimicrobial agents as proposed by the Centers for Disease Prevention and Control (CDC) and European CDC11. It was found that 24 (48%) isolates were multidrug resistant.
Discussion
In a country like India where there is high morbidity due to diarrhoeal diseases, it is pertinent to identify the true cause of diarrhoea and target the particular organism for treatment. Aeromonas is present ubiquitously in the environment and presents itself to multiple opportunities for human contact. Before it was established as an aetiologic agent in human infections, it was well known as a cause of soft tissue infections and septicaemia in aquatic animals. Aeromonas as an enteropathogen has received a lot of controversies. According to Janda and Abbott5, for an organism to be labelled as enteropathogenic, it should have led to clonal outbreaks and a source of outbreak should be established. Aeromonas is a normal habitant of aquatic environments, fresh as well as salty estuarine waters5. Multiple species may also exist in a single environment, making it difficult to establish the common source. Infections are believed to be acquired through contaminated water or food items indirectly on contact with contaminated irrigation water or seafood due to their filter feeding mechanism5. Only a few outbreaks of Aeromonas-related gastroenteritis are reported12. From our centre, a unique outbreak of hospital-acquired diarrhoea due to Aeromonas sobria was reported in 2004 affecting six children in a haemato-oncology unit13. These isolates had similar biotypes and antibiograms; however, molecular typing could not be performed. In a review of Aeromonas-associated diarrhoea by von Graevenitz12, a causal role of Aeromonas in gastroenteritis could not be established due to high degree of co-infections reported and lack of clonality in outbreak clinical and environmental isolates. It is, therefore, important that more evidence is available to establish causation. In the present study, over 15 yr, 1595 cases were clinically suspected of cholera, of whom 50 (3.14%) were found to be due to Aeromonas species. This may be an underestimation of the overall incidence of Aeromonas-related gastroenteritis as we studied its role in causation of cholera-like illness only, in the setting of ongoing cholera outbreaks. In a prospective study over two years conducted in two tertiary care centres in Kolkata, India, 3.1 and 6.5 per cent of their total diarrhoea cases had Aeromonas isolated from them, amounting to a total of 164 cases14. About 42 per cent of these cases had co-infections with other pathogens. In our study, only in two cases, there were other pathogens identified i.e. S. sonnei and Salmonella enterica serotype Senftenberg. The estimates of asymptomatic carriage range between 1 and 4 per cent overall and <1 per cent in industrialized countries5151617. Aeromonas is known to infect immunocompetent and immunocompromised individuals of all age groups5. However, some studies specify adults as a vulnerable population more than children1218. In our study, maximum infection was found in children aged under-five (36% of total) followed by 16-25 age group (24%). This pattern did not change even after excluding the six cases of hospital-acquired diarrhoea previously reported13. According to the GEMS study also, Aeromonas ranks among the top five most common enteropathogens causing childhood diarrhoea in developing countries2. We also observed that the isolation rates were maximal in the warmer months of the year that is July-August, similar to other studies1218.
Ability of Aeromonas to cause cholera-like disease with typical rice water stools has been rarely reported and is limited to a handful of case reports51920. These cases range in age from two year old to 67 yr old patients. However, in our study, there were 13 patients with typical rice water stools and severe dehydration. In the Kolkata study, 44.9 per cent of 78 patients were reported to have severe dehydration although the typical stool character was not described. In our study, one of the patients died shortly following consumption of seafood at a party, which was likely the source of Aeromonas isolated from his stool. He had severe dehydration followed by hypotension, shock and death. More prospective studies focussed on this grave presentation may further support this important finding.
The identification of the organism often requires a high index of suspicion with the inclusion of selective medium in the routine identification scheme for stool pathogens. We introduced 5 per cent sheep blood agar into the conventional bacterial identification scheme for stool samples to isolate Aeromonas to increase the sensitivity of isolation of even ampicillin susceptible strains. It was found that 14 per cent of our isolates, none of which were Aeromonas trota, were susceptible to amoxicillin. This has been reported in other studies also confirming that some isolates are missed using ampicillin blood agar as a medium for selective isolation of Aeromonas2122. Moreover, 35 per cent of A. caviae has been reported to be susceptible to ampicillin8. The species frequency isolated from diarrhoea cases is similar to other studies wherein A. caviae and A. veronii remain the most common species isolated2324. A. hydrophila, which is the most common species reported in clinical cases overall, more often causes septicaemia and wound infections2526. There is a certain difference in the species distribution according to the geographical area also. In Australia and Thailand, A. hydrophila was the most common species whereas, in Europe and Americas, A. caviae is most common27. In the Kolkata study also, A. caviae was found to be the most common species of Aeromonas causing gastroenteritis14.
It is important to recognize the presence of multidrug resistance in this organism. In our study 48 per cent of isolates were multidrug resistant, similar to the Kolkata study and in contrast to a study from Western Australia1421. In the current study, a high-level resistance to quinolones and co-trimoxazole was observed. In a previous report from our centre also, 36 per cent ciprofloxacin resistance was reported in 14 Aeromonas isolates28. In Western countries such as Spain and Australia, fluoroquinolone resistance was much less seen although nalidixic acid resistance of 41 per cent was reported in a Spanish study in clinical isolates of Aeromonas29. In the Kolkata study, 12-22 per cent resistance to ciprofloxacin was reported and the mechanism involved was shown to be mutations in the quinolone resistance-determining region of gyrA and parC1430. Resistance to third-generation cephalosporins was 24 per cent which was similar to the previous report in 2004 from our centre, but in contrast to Western countries, where no or low-level resistance was seen to third- and fourth-generation cephalosporins212829. Macrolides such as azithromycin are viable treatment options to which the organism was seen to be uniformly susceptible. Cholera being one of the most common causes of bacterial diarrhoea, cases with classical symptoms of acute watery stools are often assumed to be cholera and treated with ciprofloxacin to which they are more or less uniformly susceptible although, in the last decade, fluoroquinolone resistance is emerging in cholera3132.
The importance of Aeromonas as a gastrointestinal pathogen must be recognized, and therefore, it must be actively sought in a diarrhoea case.
Conflicts of Interest: None.
References
- Systematic review of diarrhea duration and severity in children and adults in low- and middle-income countries. BMC Public Health. 2012;12:276.
- [Google Scholar]
- Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382:209-22.
- [Google Scholar]
- Molecular epidemiology of Vibrio cholerae causing outbreaks and sporadic cholera in Northern India. Indian J Med Res. 2012;136:656-63.
- [Google Scholar]
- The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23:35-73.
- [Google Scholar]
- Aeromonas species in septicemia: Laboratory characteristics and clinical observations. Clin Infect Dis. 1994;19:77-83.
- [Google Scholar]
- Aerokey II: A flexible key for identifying clinical Aeromonas species. J Clin Microbiol. 1991;29:2843-9.
- [Google Scholar]
- Manual of clinical microbiology (10th ed). Washington, DC: ASM Press; 2011.
- Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493-6.
- [Google Scholar]
- Performance Standards for Antimicrobial Susceptibility Testing;Twenty-First Informational Supplement M100-S21. Wayne, PA: CLSI; 2011.
- Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268-81.
- [Google Scholar]
- An outbreak of hospital acquired diarrhea due to Aeromonas sobria. Indian Pediatr. 2004;41:912-6.
- [Google Scholar]
- Prevalence, serotype distribution, antibiotic susceptibility and genetic profiles of mesophilic Aeromonas species isolated from hospitalized diarrhoeal cases in Kolkata, India. J Med Microbiol. 2004;53(Pt 6):527-34.
- [Google Scholar]
- Aeromonas in adult diarrhea: An enteropathogen or an innocent bystander? J Clin Gastroenterol. 1990;12:148-52.
- [Google Scholar]
- Aeromonas hydrophila and Plesiomonas shigelloides as causes of intestinal infections. Rev Infect Dis. 1984;6:633-9.
- [Google Scholar]
- Aeromonads in acute diarrhoea and asymptomatic infections in Nigerian children. Eur J Epidemiol. 1995;11:171-5.
- [Google Scholar]
- Aeromonas species and Plesiomonas shigelloides in diarrhoea in Goa. Indian J Pathol Microbiol. 1995;38:169-71.
- [Google Scholar]
- Cholera-like diarrhea in Canada. Report of a case associated with enterotoxigenic Escherichia coli and a toxin-producing Aeromonas hydrophila. Arch Intern Med. 1977;137:1461-4.
- [Google Scholar]
- Antimicrobial susceptibilities of Aeromonas strains isolated from clinical and environmental sources to 26 antimicrobial agents. Antimicrob Agents Chemother. 2012;56:1110-2.
- [Google Scholar]
- Sampling bias created by ampicillin in isolation media for Aeromonas. Can J Microbiol. 2007;53:39-44.
- [Google Scholar]
- Aeromonas spp. and traveler's diarrhea: Clinical features and antimicrobial resistance. Emerg Infect Dis. 2003;9:552-5.
- [Google Scholar]
- Distribution, virulence-associated genes and antimicrobial resistance of Aeromonas isolates from diarrheal patients and water, China. J Infect. 2015;70:600-8.
- [Google Scholar]
- Clinical manifestations of bacteremia caused by Aeromonas species in Southern Taiwan. PLoS One. 2014;9:e91642.
- [Google Scholar]
- Skin and soft-tissue infections caused by Aeromonas species. Eur J Clin Microbiol Infect Dis. 2013;32:543-7.
- [Google Scholar]
- Antimicrobial resistance in selected bacterial enteropathogens in North India. Indian J Med Res. 2004;120:39-43.
- [Google Scholar]
- In vitro antimicrobial susceptibility of clinical isolates of Aeromonas caviae, Aeromonas hydrophila and Aeromonas veronii biotype sobria. J Antimicrob Chemother. 2002;49:701-2.
- [Google Scholar]
- An unusually high level of quinolone resistance associated with type II topoisomerase mutations in quinolone resistance-determining regions of Aeromonas caviae isolated from diarrhoeal patients. Res Microbiol. 2004;155:827-9.
- [Google Scholar]
- Genetic characteristics of drug-resistant Vibrio cholerae O1 causing endemic cholera in Dhaka 2006-2011. J Med Microbiol. 2012;61(Pt 12):1736-45.
- [Google Scholar]






