Translate this page into:
Evaluating KIND1 human embryonic stem cell-derived pancreatic progenitors to ameliorate streptozotocin-induced diabetes in mice
Reprint requests: Dr. Deepa Bhartiya, Department of Stem Cell Biology, ICMR-National Institute for Research in Reproductive Health, Jehangir Merwanji Street, Parel, Mumbai 400 012, Maharashtra, India e-mail: bhartiyad@nirrh.res.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Diabetes is a global disease burden. Various stem cell types are being explored to serve as an alternative source of islets. This study was conducted to evaluate the ability of in-house developed human embryonic stem (hES) cells-derived pancreatic progenitors to ameliorate diabetic symptoms in mice.
Methods:
Pancreatic progenitors were packed in macro-capsules and transplanted into six male Swiss mice and four mice were taken as controls. Thirty days post-transplantation, diabetes was induced by streptozotocin treatment. Mice were then followed up for >100 days and body weight and blood glucose levels were regularly monitored.
Results:
Control mice lost weight, maintained high glucose levels and did not survive beyond 40 days, whereas transplanted group maintained body weight and four of the six mice had lowered blood glucose levels. About five-fold increase was observed in human C-peptide levels in the recipients of progenitor transplants as compared to diabetic control.
Interpretation & conclusions:
The beneficial effect of transplanted cells was not long-lasting. Further studies are required to critically evaluate and compare the potential of endogenous pluripotent stem cells and hES cells-derived progenitors before moving from bench to the bedside.
Keywords
Diabetes
differentiation
hESC
pancreas
progenitors
stem cells
transplantation
Currently, 415 million people suffer from diabetes worldwide compared to 151 million in 2000, with an expected rise to 642 million by 2040. Every sixth second, a person dies from diabetes1. The first report of reversal of diabetes as a part of Edmonton protocol using cadaveric islets in 2000 raised hopes for the treatment of this debilitating disease. However, the scarcity of pancreas and cadaveric islet donors has made stem cells a promising alternative cell source to differentiate into islets for treating diabetes2.
Because of their ability to self-renew indefinitely, human embryonic stem cells (hESC) could be an ideal source of islets in vitro and may allow treatment of a large number of patients. D’Armor and colleagues3 first reported the differentiation of hES cells into pancreatic progenitors, later placed the differentiated cells overlaid on a scaffold followed by transplantation in SCID mice and detected human insulin and C-peptide release4, developed scalable system for producing functional progenitors and documented the efficiency of their product PEC-015. Similarly, in another study 30 per cent of transplanted mice showed reduction in hyperglycaemia on transplanting insulin positive cells obtained by differentiating ES cells, for over a period of six months6. Bruin et al7 improved the differentiation protocol further which resulted in grafts containing >80 per cent endocrine cells and resulted in single hormonal cells expressing either insulin or glucagon or somatostatin in contrast to earlier polyhormonal cells. Kirk et al8 have demonstrated that human insulin is secreted by seven weeks after transplantation of encapsulated pancreatic progenitors, and by 20 wk, enough human insulin is produced to ameliorate alloxan-induced diabetic symptoms. The differentiated endocrine cells were monohormonal and insulin was produced in response to a glucose challenge.
The current research efforts are focussed on optimizing the encapsulation procedures of transplanted hES-derived progenitors. Vegas et al9 achieved normoglycaemia upto 174 days on using triazole-thiomorpholine dioxide (TMTD)-coated alginate microspheres where TMTD resists fibrosis on the surface. Modifications of existing protocols by including scale-up, enrichment and maturation of islet-like cells before transplantation, have also been reported5.
Cell lines developed by our group (KIND1 and KIND2) were derived on human feeders10 and were evaluated for their propensity to develop into various germ layers11. These were later adapted to feeder-free conditions and differentiated into pancreatic progenitors12 using published protocols. The present study was undertaken to evaluate the feasibility, efficacy and safety of pancreatic progenitors developed from KIND1 hES cells which had earlier shown a propensity to form endoderm. The cells were encapsulated in biocompatible macro-capsules for intra-peritoneal transplantation in mice as reported earlier13. Mice were later treated with streptozotocin (STZ) and followed up long-term to evaluate functional maturation of islets and their ability to lower blood glucose levels.
Material & Methods
The study was undertaken in Stem Cell Biology department of the ICMR-National Institute for Research in Reproductive Health, Mumbai, India. All chemicals for cell culture were obtained from Life Technologies (Carlsbad, CA, USA) unless otherwise indicated. The study was approved by the Institute Committee for Stem Cells Research and Animal Ethics Committee.
Human embryonic stem (hES) cells culture: hES cells (KIND1) were adapted to feeder-free conditions1012. Cells were cultured and expanded in 60 mm Petri dish coated with Geltrex using StemPro hESC serum-free medium supplemented with 8 ng/ml of basic fibroblast growth factor (FGF) (R&D Systems, MN, USA) at 37°C in a humidified atmosphere with 5 per cent CO2. Passaging was done mechanically using cell lifter (Sigma-Aldrich, MO, USA) in 1:3 ratio.
In vitro differentiation of hESC: hES cells were differentiated into endoderm lineage as described earlier12. Briefly, feeder-free hES cells showing 80 per cent confluency were cultured in RPMI 1640 medium containing 100 ng/ml activin A (R&D Systems), 1mM sodium butyrate (Sigma-Aldrich), and 25 ng/ml Wnt-3a (R&D Systems). After 24 h, 0.2 per cent foetal bovine serum (FBS) was added to RPMI media along with 100 ng/ml activin A, 0.5 mM sodium butyrate. On days 3 and 4, the RPMI medium was supplemented with 2 per cent FBS and 100 ng/ml activin A. From day 4 onwards, the basal medium used was DMEM-F12 supplemented with 1×B27, 2 μM retinoic acid (Sigma-Aldrich), 50 ng/ml noggin (R&D Systems), 0.25 μM cyclopamine (Sigma-Aldrich) for four days. During the last stage of differentiation protocol, the cells were cultured in DMEM along with 1×B27 supplement, 2 μM retinoic acid, 50 ng/ml noggin, 1mM nicotinamide (Sigma-Aldrich), 1× non-essential amino acids and 25 μg/ml FGF-10 (R&D Systems).
Characterization of hESC and differentiated pancreatic progenitors by specific markers: The KIND1 hES cells grown in feeder-free conditions maintained pluripotent characteristics (expressing OCT4, NANOG, SOX2, REX1, TERT, SSEA4) and differentiated into pancreatic progenitors which were further characterized at both protein and mRNA levels by immunofluorescence (SOX9, SOX17, PDX1) and quantitative reverse transcription polymerase chain reaction (qRT-PCR, SOX9, SOX17, NKX6.1, PDX1) studies using specific markers. qRT-PCR studies were also undertaken to detect the presence of mesoderm (MESP1 and NKX2.5) and ectoderm (SOX1 and MAP2)-specific cell types among the pancreatic progenitors obtained by directed differentiation.
Immunofluorescence: The KIND1 cells were fixed with freshly prepared 4 per cent paraformaldehyde and washed thrice with phosphate buffered saline (PBS) and 0.02 per cent Tween 20 (Sigma-Aldrich). For intracellular antigens, permeabilization was done with 0.3 per cent Triton X-100 (Sigma-Aldrich). Blocking of non-specific sites was carried out with blocking buffer composed of 5 per cent bovine serum albumin (Sigma-Aldrich) and one per cent normal goat serum (Bangalore Genei, Bangalore) in PBS for 60 min at room temperature. The cells were incubated overnight at 4°C with primary antibodies against rabbit anti- SOX9 (1:200, Millipore), rabbit anti-SOX17 (1:200, Millipore) and rabbit anti-PDX1 (1:100, Epitomics CA, USA). After three washes, the cells were incubated with goat Alexa Fluor 488 (Molecular Probes, Invitrogen, USA) secondary antibody diluted (1:1000) in blocking buffer for two hours at room temperature. The cells were washed, counterstained with 300nM DAPI (Molecular Probes, Invitrogen) and photographed under confocal microscope (Carl Zeiss, Germany).
Quantitative reverse transcription polymerase chain reaction (qRT-PCR): Total RNA was extracted using TRIzol reagent (Invitrogen) as per the manufacturer's instructions. Spectrophotometric quantification of the extracted RNA was done using Ultrospec 3100 Pro (GE Healthcare, PA, USA). The cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, CA, USA) in a 20μl reaction volume according to the manufacturer's instructions using G-STORM thermal cycler (Gene Technologies, Braintree, UK). qRT-PCR was performed using CFX96 Real-Time Machine (Bio-Rad, CA, USA) and iQ SYBR Green SuperMix (Bio-Rad). The threshold values (Ct) were obtained from CFX 96 manager software (Bio-Rad) and normalized using housekeeping gene GAPDH. The primer sequences121415 are given in Table I. The amplification conditions comprised initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 10 sec, annealing at 62°C for 20 sec and elongation at 72°C for 30 sec. The fold change in expression was calculated by 2−ΔΔCt method. Each reaction was carried out in duplicate. The expression of gene transcripts specific for definitive endoderm (day 4), pancreatic gut tube (day 8), and pancreatic progenitor (days 12-16) is expressed relative to undifferentiated hES cells.
Transplantation studies in mice: Initially, establishment of model, its characterization and developing methods to transplant capsules were undertaken using several batches of mice. This study was undertaken using 10-12 wk old male mice, control (n=4) and transplanted (n=6). Macro-encapsulated day 16 derived pancreatic progenitors were transplanted intraperitoneally into six male Swiss mice. After 30 days of transplantation, all mice were treated with STZ and 15 days later were maintained on human insulin (1 IU/kg) given subcutaneously. Human insulin was discontinued after two weeks and mice were regularly monitored for their weight and blood glucose levels for a period of 110 days. They were carefully monitored for their body weight and blood glucose levels. Glucose was measured in blood collected from tail vein using contour glucometer.
Encapsulation and transplantation of hES-derived pancreatic progenitors: For transplantation of pancreatic progenitors, biocompatible macro-capsules made up of polyurethane-polyvinyl pyrrolidone semi-interpenetrating network, were employed16. The encapsulation procedure was carried out in strictly aseptic conditions as described earlier13. Confluent hES cells differentiated into pancreatic progenitors were detached mechanically from culture plates using a cell lifter, centrifuged and re-suspended into 300 μl basal medium (DMEM-F12). This cell suspension was transferred into the capsules and the open ends were sealed using a heated forceps. Transplanted group animals were anaesthetized using xylazine (20 mg/kg) and ketamine (80 mg/kg) and approximately 0.8-1.0×106 pancreatic progenitor cells encapsulated in the biocompatible device were transplanted in the peritoneal cavity of each of the six mice. Post-transplantation animals were maintained on a healthy diet.
Streptozotocin (STZ) treatment and follow up studies: After 30 days of transplantation, all mice (n=10) were rendered diabetic with multiple low-dose STZ (50 mg/kg) injections given for four consecutive days. Body weight and blood glucose were monitored daily post-STZ treatment. Once the blood glucose levels reached >300 mg/dl, all the mice received exogenous insulin support (1 IU/kg, subcutaneously) for 15 consecutive days. Animal survival, overall health, body weight and blood glucose levels were monitored at regular intervals for the next 120 days.
ELISA for human C-peptide: C-peptide levels were measured to assess further differentiation and maturation of the transplanted pancreatic progenitor cells. Blood (30 μl each) was collected from one control and two transplanted animals by retro-orbital bleeding, centrifuged and serum was collected for ELISA for human C-peptide as per the manufacturer's instruction (EZHCP-20K, Merck Millipore, Germany). Secreted C-peptide levels were quantified using BioTek plate reader (BioTek Instruments, VT, USA).
Results
Human embryonic stem cells culture: Undifferentiated hES cell line KIND1 was grown and maintained in feeder-free and monolayer condition (Fig. 1A and B). Feeder-free KIND1 cells were regularly characterized at transcript and protein level by qRT-PCR and immunofluorescence for the maintenance of their pluripotent state using markers such as OCT4, NANOG, SOX2, REX1, TERT and SSEA4 (Fig. 1C and D).
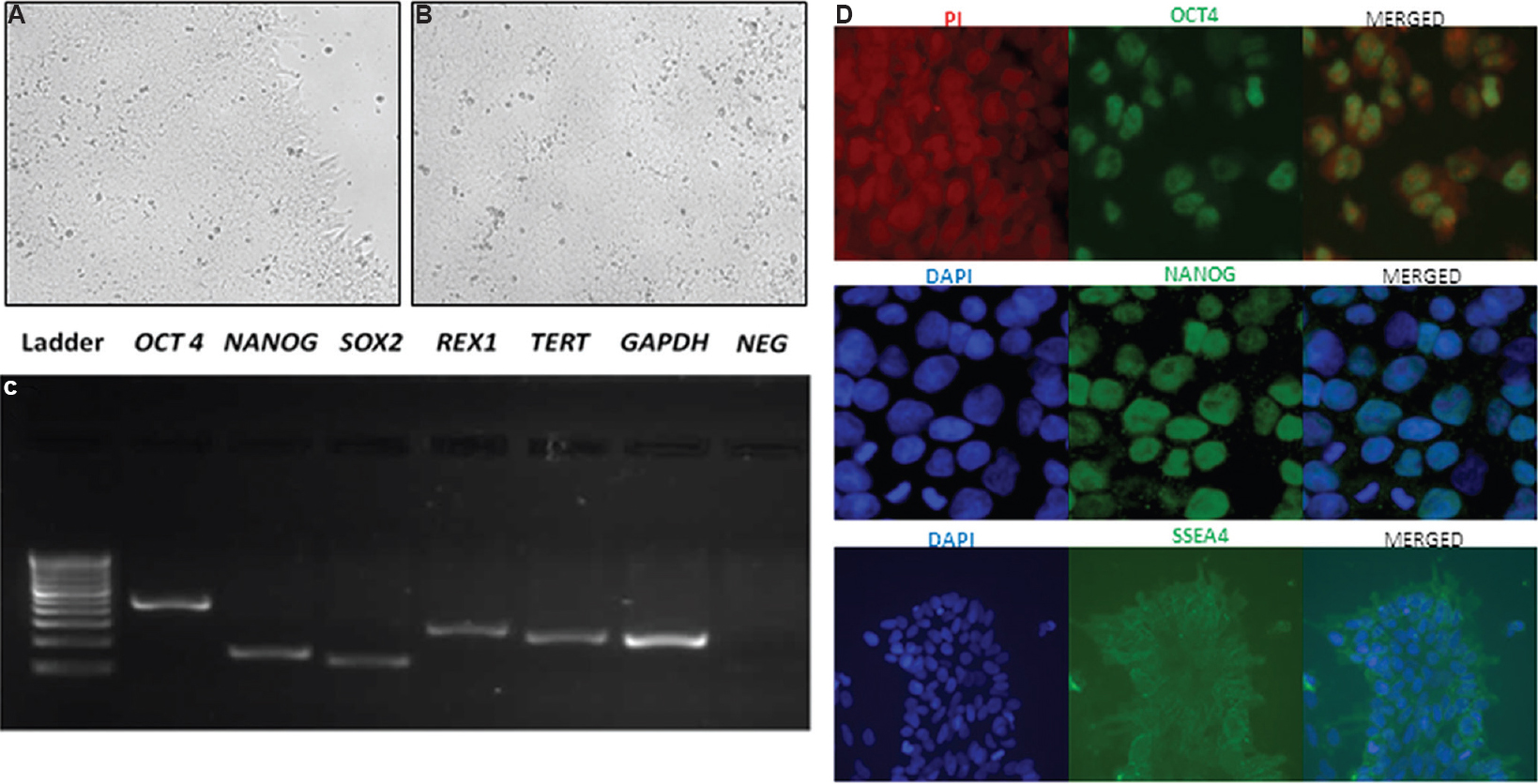
- Human embryonic stem cells (KIND1) used for the study. (A & B) KIND1 human embryonic stem cells colony grown as monolayer under feeder-free conditions on a Geltrex-coated dish (×10). (C) Pluripotent transcripts amplified by reverse transcription polymerase chain reaction using KIND1 human embryonic stem cells OCT4 (octamer-binding transcription factor 4), NANOG, SOX2 (SRY-Box 2), REX1 (Reduced Expression Protein 1) and TERT (Telomerase Reverse Transcriptase). GAPDH was used as housekeeping gene. (D) Immunofluorescence staining revealed the localization of markers such as OCT4, NANOG and SSEA4 (stage-specific embryonic antigen-4) (×40) (top & middle panel) and ×20 (bottom panel). Counterstaining was done using PI for OCT4 while DAPI was used for NANOG and SSEA4.
In vitro differentiation of hESC: Our optimized directed differentiation protocol for endoderm differentiation from hES cells led to the formation of pancreatic progenitors by day 16 (Fig. 2A). During differentiation, sequential addition of growth factors led to the appearance of transcripts specific to the developing endoderm lineage.
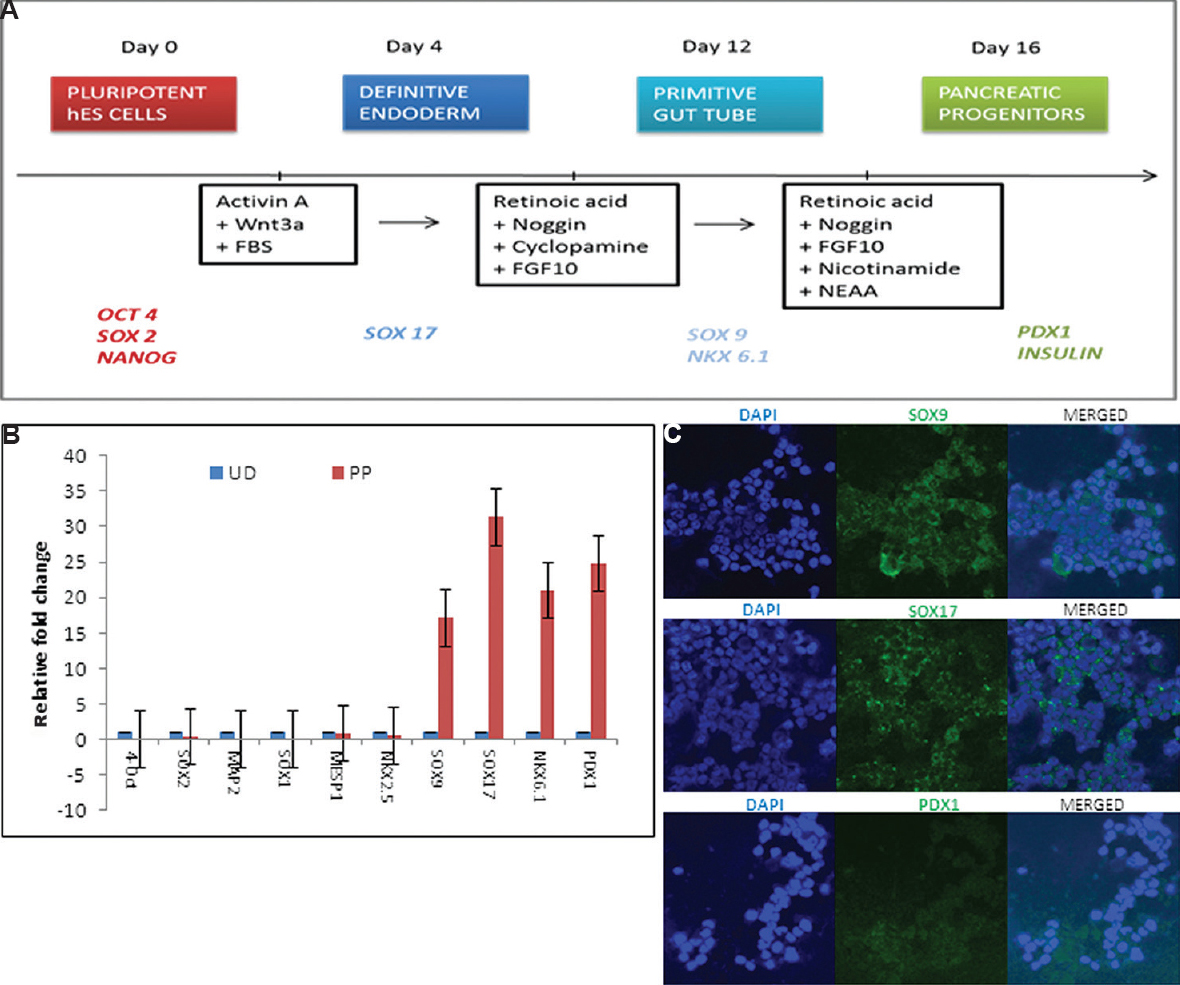
- Differentiation of KIND1 cells into pancreatic progenitors. (A) Schematic representation of protocol. (B) Expression of pluripotency (OCT4, SOX2), pancreatic progenitor-specific (SOX17 (SRY-Box 17), SOX9 (SRY-Box 9), NKX6.1 (NK6 homeobox 1), PDX1 (Pancreas/duodenum homeobox protein 1), ectodermal SOX1 (SRY-Box 1), MAP2 (Microtubule-associated protein 2) and mesodermal MESP1 (mesoderm posterior bHLH transcription factor 1), NKX2.5 (NK2 homeobox 5) transcripts in undifferentiated (UD, blue) and pancreatic progenitors on day 16 (PP, red). Data represent mean±standard error of the mean. (C) Immunofluorescence for SOX9, SOX17 and PDX1 (×10).
Characterization of differentiated pancreatic progenitors: Formation of endoderm lineage from hES cells was evident by detection of genes such as SOX17 and SOX9 marking the formation of definitive endoderm and primitive gut tube, respectively, as well as NKX6.1 and PDX1 representing the formation of pancreatic progenitors. This was supported by significant downregulation of pluripotent markers OCT4A and SOX2 along with low levels of ectoderm- and mesoderm-specific genes such as SOX1, MAP2 and MESP1, NKX2.5, respectively, as compared to undifferentiated feeder-free hES cells (Fig. 2B). Immunofluorescence studies of hES cell-derived pancreatic progenitors also revealed the formation of pancreatic endoderm lineage as evident by the localization of the specific markers such as SOX9, SOX17 and PDX1 by confocal microscopy (Fig. 2C).
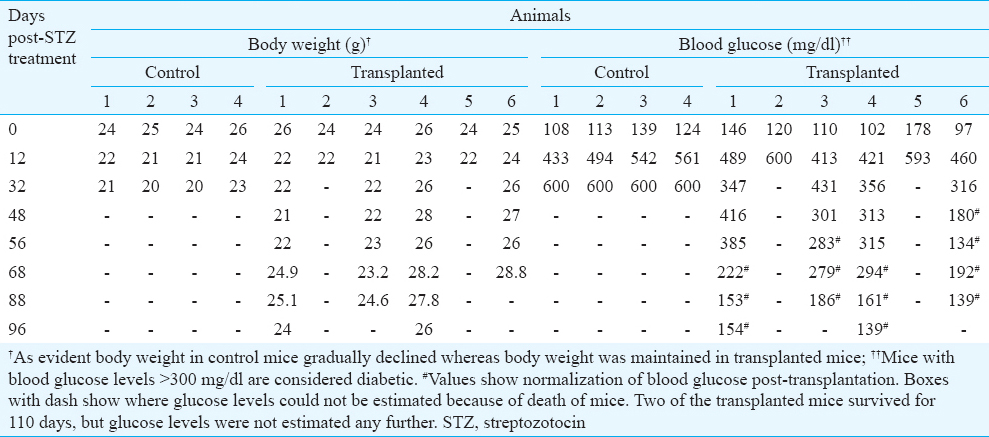
Transplantation studies in mice: Approximately 7-8 weeks post-STZ treatment, blood glucose level in four out of six experimental animals started decreasing and achieved normal levels (Table II). Normalization of hyperglycaemia was accompanied with an increase in body weight (Table II). Two mice in the experimental group died on days 20 and 32, respectively, post-STZ. On the other hand, diabetic control animals (n=4) maintained high blood glucose levels and gradual weight loss throughout the study and were dead by day 45 of STZ treatment (Table II). The remaining transplanted mice continued with lowered blood glucose level and normal body weight for 4-5 weeks, after which the experiment was terminated due to ill health of the mice.
ELISA for human C-peptide: As a measure of development and functionality of transplanted progenitors, human C-peptide levels were analyzed on day 100 of experiment in two of the experimental animals (1.99 and 1.45 ng/ml) compared to that of diabetic control animal (0.37 ng/ml). More than five folds increased release in human C-peptide levels were noticed in the recipients of progenitor transplants as compared to diabetic control. Similar results were obtained in a smaller experiment done earlier transplanting pancreatic progenitors in four mice.
Discussion
The present study attempted to assess the effect of hES cells derived pancreatic progenitors in diabetic mouse model. Pancreatic progenitors obtained by in vitro differentiation of KIND1 hES cells10 were packed in an immunoisolatory device and transplanted in mice. One month later the mice were made diabetic and the transplanted progenitors evidently became functional after another two months (3 months post-transplantation) and helped to maintain low blood glucose levels and body weight for a period of 4-5 weeks. The time taken by the progenitor cells to become functional was in agreement to the in vivo maturity period17, and also in agreement with another published report4. C-peptide estimation is an indirect measurement of human insulin in circulation. The progenitors had the ability to further mature into beta islets as shown by secretion of human C-peptide in mouse circulation. It was feasible to transplant the progenitors, achieve full maturation into islets in mice and the approach was found to be safe since no teratoma was observed in any of the transplanted mice. However, the study was terminated by day 110 because the mice were sick and would not have survived any longer. This could be a limitation of the model or might be due to poor efficiency of differentiation of hES cells into pancreatic progenitors or maturation post-transplantation.
Based on the work published from our laboratory31819, we were keen to compare the potential of endogenous pluripotent stem cells to regenerate a diabetic pancreas with hES cells-derived pancreatic progenitors (grown in a Petri dish). The analyses of various published pre-clinical studies describing the outcome of transplanted pancreatic progenitors (Table III) suggest that ES cells have the potential to differentiate into islets and human C-peptide and insulin are detected in circulation. Majority of studies were for 120-175 days including the present study and only one study21 followed up mice for 238 days. This group reported that the pancreatic progenitors in vivo exhibited gene and protein expression profiles remarkably similar to the developing human foetal (not adult) pancreas. Jiang and Morahan26 have concluded that although ES/induced pluripotent stem (iPS) theoretically has the ability to differentiate into functional beta cells, the field has not advanced as expected.

While studying the epigenetic changes involved during differentiation of ES cells into pancreatic progenitors, we have earlier reported that polycomb group proteins including both PRC1 (RING1, BMI1, CBX) and PRC2 (SUZ12, EED, EZH2) specific transcript levels are different in D16 progenitors compared to adult pancreas212. These differences may get ameliorated when the progenitors differentiate post-transplantation into mature islets or this may be the basic underlying cause which results in foetal-like state of ES-derived progenitors in STZ-treated mice and prevent their further differentiation into the adult state. We postulate that these epigenetic differences between ES-derived progenitors compared to adult human pancreatic cells are of significance and further careful studies need to be undertaken to address this in details.
A careful review of the literature reveals that even though several groups have attempted to differentiate both mouse and hES/iPS cells into gametes, research has not progressed as expected. The main reason is the inefficient conversion of ES cells into primordial germ cells (PGCs) which is the first and most crucial step to convert ES cells into gametes27. ES cells are derived by in vitro expansion of the inner cell mass of blastocyst stage embryo, whereas PGCs are developmentally more mature cells with a distinct epigenetic profile which appear in the yolk sac of developing epiblast stage embryo and migrate towards the gonadal ridge where they differentiate into gametes. Thus, by closely monitoring hES cells differentiation into pancreatic progenitors and gametes, it is postulated that various differentiation protocols transition hES cells into desired cell types at genetic level but fall short at epigenetic level and thus remain in foetal state and may not mature to an adult state (which is crucial for effective and long-term regeneration). This may be a major limitation for use of hES/iPS cells for regenerative medicine. Various adult cell types are also being used to regenerate a diabetic pancreas with varying results including autologous bone marrow cells and mesenchymal cells2. Autologous bone marrow cells failed to benefit ST elevation in cases of acute myocardial infarction in a multi-centric clinical trial concluded in India28. Bhansali et al29 reported beneficial effect of transplanting mesenchymal cells through the tail vein in diabetic mice, but whether the beneficial effect was due to mesenchymal stem cells or very small embryonic-like stem cells (VSELs) needs further clarification30.
The best strategy to alleviate the disease symptoms will possibly be to manipulate endogenous stem cells in the diabetic pancreas31. Various mechanisms have been proposed to explain regeneration of pancreas. It has earlier been demonstrated that VSELs are present in mouse pancreas and get mobilized in large numbers after STZ treatment in mice and also during pancreatic cancers in humans32. Two groups have reported distinct OCT-4 positive, small-sized cells in the human pancreas3334. The small size and being present in very few numbers have led to the non-detection of VSELs35 during otherwise a very good lineage tracing study36. We have recently observed VSELs involvement in regenerating a diabetic mouse (STZ treated) pancreas after partial pancreatectomy18. Preliminary data from our lab shows that when STZ treated mice were subjected to partial pancreatectomy, they remained healthy and maintained normal body weight and normoglycaemia for more than six months (unpublished observations).
To conclude, the potential of endogenous pluripotent stem cells (VSELs) to regenerate a diabetic pancreas needs to be compared with hES cells-derived pancreatic progenitors (grown in a Petri dish) to develop strategies to increase the effective span of cell therapy.
Acknowledgment
Authors acknowledge the Department of Biotechnology, Government of India, New Delhi, for funding the initial derivation of ES cell lines and their differentiation.
Conflicts of Interest: None.
References
- International Diabetes Federation (IDF). IDF diabetes atlas (7th ed). Brussels, Belgium: IDF; 2014.
- Stem cells to replace or regenerate the diabetic pancreas: Huge potential & existing hurdles. Indian J Med Res. 2016;143:267-74.
- [Google Scholar]
- Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392-401.
- [Google Scholar]
- Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo . Nat Biotechnol. 2008;26:443-52.
- [Google Scholar]
- Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo . Stem Cells Transl Med. 2015;4:1214-22.
- [Google Scholar]
- In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333-44.
- [Google Scholar]
- Maturation and function of human embryonic stem cell-derived pancreatic progenitors in macroencapsulation devices following transplant into mice. Diabetologia. 2013;56:1987-98.
- [Google Scholar]
- Human embryonic stem cell derived islet progenitors mature inside an encapsulation device without evidence of increased biomass or cell escape. Stem Cell Res. 2014;12:807-14.
- [Google Scholar]
- Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med. 2016;22:306-11.
- [Google Scholar]
- Derivation and characterization of two genetically unique human embryonic stem cell lines on in-house-derived human feeders. Stem Cells Dev. 2009;18:435-45.
- [Google Scholar]
- Evaluating differentiation propensity of in-house derived human embryonic stem cell lines KIND-1 and KIND-2. In Vitro Cell Dev Biol Anim. 2011;47:406-19.
- [Google Scholar]
- Polycomb group protein expression during differentiation of human embryonic stem cells into pancreatic lineage in vitro . BMC Cell Biol. 2014;15:18.
- [Google Scholar]
- Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010;7:168-82.
- [Google Scholar]
- Lineage specific expression of Polycomb Group Proteins in human embryonic stem cells in vitro . Cell Biol Int. 2015;39:600-10.
- [Google Scholar]
- Differentiation of human ES cell line KIND-2 to yield tripotent cardiovascular progenitors. In Vitro Cell Dev Biol Anim. 2013;49:82-93.
- [Google Scholar]
- A Process for the Preparation of a Biocompatible, Polymeric Composition of an Interpenetrating Polymeric Network (IPN). Indian Patent No. 230740. Filed by Sree ChitraTirunal Institute for Medical Sciences and Technology, Kerala. 2009
- [Google Scholar]
- Immunohistochemical characterization of insulin, glucagon, PDX1, SOX17 and NGN3 expression in human fetal pancreatic development. J Stem Cell Res Ther. 2013;3:148.
- [Google Scholar]
- Very small embryonic-like stem cells are involved in regeneration of mouse pancreas post-pancreatectomy. Stem Cell Res Ther. 2014;5:106.
- [Google Scholar]
- Very small embryonic-like stem cells are involved in pancreatic regeneration and their dysfunction with age may lead to diabetes and cancer. Stem Cell ResTher. 2015;6:96.
- [Google Scholar]
- Inconsistent formation and non-function of insulin-positive cells from pancreatic endoderm derived from human embryonic stem cells in athymic nude rats. Am J Physiol Endocrinol Metab. 2010;299:E713-20.
- [Google Scholar]
- Enrichment of human embryonic stem cell-derived NKX6.1-expressing pancreatic progenitor cells accelerates the maturation of insulin-secreting cells in vivo . Stem Cells. 2013;31:2432-42.
- [Google Scholar]
- Transplantation of human embryonic stem cell-derived pancreatic endoderm reveals a site-specific survival, growth, and differentiation. Cell Transplant. 2013;22:821-30.
- [Google Scholar]
- Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121-33.
- [Google Scholar]
- Generation of functional human pancreatic β cells in vitro . Cell. 2014;159:428-39.
- [Google Scholar]
- Pancreatic islet-like three-dimensional aggregates derived from human embryonic stem cells ameliorate hyperglycemia in streptozotocin-induced diabetic mice. Cell Transplant. 2015;24:2155-68.
- [Google Scholar]
- Making gametes from pluripotent stem cells - A promising role for very small embryonic-like stem cells. Reprod Biol Endocrinol. 2014;12:114.
- [Google Scholar]
- Efficacy of stem cell in improvement of left ventricular function in acute myocardial infarction – MI3 Trial. Indian J Med Res. 2015;142:165-74.
- [Google Scholar]
- Effect of mesenchymal stem cells transplantation on glycaemic profile & their localization in streptozotocin induced diabetic Wistar rats. Indian J Med Res. 2015;142:63-71.
- [Google Scholar]
- Is the improved function of streptozotocin treated pancreas truly due to transdifferentiation/fusion of transplanted MSCs? Indian J Med Res. 2016;143:111-2.
- [Google Scholar]
- Anonymous sources: Where do adult ß cells come from? J Clin Invest. 2013;123:1936-8.
- [Google Scholar]
- An intensified systemic trafficking of bone marrow-derived stem/progenitor cells in patients with pancreatic cancer. J Cell Mol Med. 2013;17:792-9.
- [Google Scholar]
- Pluripotency-associated stem cell marker expression in proliferative cell cultures derived from adult human pancreas. J Endocrinol. 2011;211:169-76.
- [Google Scholar]
- Evidence for the presence of stem cell-like progenitor cells in human adult pancreas. J Endocrinol. 2007;195:407-14.
- [Google Scholar]
- Endogenous, very small embryonic-like stem cells: Critical review, therapeutic potential and a look ahead. Hum Reprod Update. 2016;23:41-76.
- [Google Scholar]
- No evidence for β cell neogenesis in murine adult pancreas. J Clin Invest. 2013;123:2207-17.
- [Google Scholar]






