Translate this page into:
Molecular & genetic characteristics of Mycobacterium tuberculosis strains circulating in the southern part of West Siberia
Reprint requests: Dr. Oksana Pasechnik, Omsk State Medical University, 644099, Omsk, Russia e-mail: opasechnik@mail.ru
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
A complicated epidemiological situation characterized by significantly high tuberculosis (TB) morbidity is observed in West Siberia. This study was aimed to investigate the genetic characteristics of Mycobacterium tuberculosis circulating in the southern part of West Siberia (in the Omsk region).
Methods:
From March 2013 to January 2015, 100 isolates of M. tuberculosis were obtained from patients with pulmonary TB living in the Omsk region. Drug susceptibility testing was performed on Lowenstein-Jensen medium (absolute concentration method). Genetic typing of isolates was carried out by variable number tandem repeats of mycobacterial interspersed repetitive units (MIRU-VNTR) typing and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) analysis. The genetic types and characteristics of cluster strains were determined using 15 MIRU-VNTR loci.
Results:
Thirty six VNTR types were found. Twenty six (26.0%) isolates had a unique profile, and the remaining 74 were grouped in 10 clusters containing from 2 to 23 isolates. The Beijing genotype was found in 72 isolates, 61 (85.0%) of which were part of five clusters that included two large clusters containing 23 isolates. Other genetic families, such as Latin-American Mediterranean (LAM, 11.0%), S family (2.0%) and Haarlem (4.0%), were also detected. The genetic family of 11 isolates could not be determined. Six different VNTR profiles were found in these non-classified isolates. Only 16 per cent of isolates were sensitive to anti-TB drugs. The katG315 (94.8%) and rpoB531 (92.2%) mutations were identified in 77 multidrug-resistant M. tuberculosis isolates.
Interpretation & conclusions:
This study showed that the M. tuberculosis population in the Omsk region was heterogeneous. The Beijing genotype predominated and was actively spreading. The findings obtained point to the need for the implementation of more effective preventive measures to stop the spread of drug-resistant M. tuberculosis strains.
Keywords
Beijing
epidemiology
genotype
incidence
MIRU-VNTR typing
Mycobacterium tuberculosis
In 2015, 10.4 billion new cases of tuberculosis (TB) were registered, 1.8 billion people died of TB, and multidrug-resistant (MDR) forms of TB developed in 480,000 patients1. According to the World Health Organization2, 46 per cent of all TB cases and 40 per cent of all deaths due to TB occur in five countries: Brazil, the Russian Federation, India, China and South Africa, comprising the BRICS group. Each of the five BRICS countries has recorded the highest TB incidence rate among the territories of the corresponding WHO region. In 2016, 47 per cent of cases of MDR TB forms have been registered in China, India and the Russian Federation2.
The Omsk region is a large industrial region in Russia located in the southern part of West Siberia. The territory covers 141,000 square kilometres, with a population of over 1,970,000 people. Complex programmes for TB prevention have led to significant progress toward making the situation stable and decreasing morbidity and mortality rates. The current problem is the spread of TB drug-resistant forms and TB incidence among HIV-infected patients3. During 2010 to 2014, TB incidence in the Omsk region decreased by 30 per cent from 115.2 to 86.1 per 100,000 population per year. However, the incidence of TB combined with HIV increased four-fold from 2.4 to 9.7 per 100,000 people per year4, and the incidence of MDR TB increased two-fold from 3.5 to 7.6 per 100,000 people3. The results of recent studies have demonstrated that the Beijing genotype is actively circulating in most Russian regions, including Siberia. This genotype is defined by high transmissibility and virulence and is associated with multidrug resistance56. The information about the Mycobacterium tuberculosis genotypes circulating in the Omsk region is lacking. The purpose of this study was to investigate the genetic characteristics of M. tuberculosis isolates found in the southern part of West Siberia in the Omsk region.

Material & Methods
Sputum samples were collected during March 2013 to January 2015 from all those patients with pulmonary TB who lived in the Omsk region and were admitted to Clinical TB Dispensary at Omsk, Russia. The age range of the patients was 19-78 years. The inclusion criteria were pulmonary TB, residency in the Omsk region, being treated in TB hospitals, age over 18, and consent to participate in the study. The exclusion criteria were as follows: (i) age less than 18 yr, (ii) extrapulmonary TB, (iii) absence of bacterial excretion, and (iv) patient refused to participate in the study.
The study was conducted in the department of Epidemiology, Omsk State Medical University (Omsk), Institute of Chemical Biology and Fundamental Medicine, SB RAS (Novosibirsk) and Omsk Clinical TB Dispensary. Isolation of M. tuberculosis, their identification and assessment of their sensitivity were performed in the Bacteriological Laboratory of Omsk Clinical TB Dispensary. DNA extraction, molecular identification of mycobacteria and variable number tandem repeats of mycobacterial interspersed repetitive units VNTR - typing were conducted in the Laboratory of Pharmacogenomics, Institute of Chemical Biology and Fundamental Medicine, SB RAS (Novosibirsk). This study was approved by the Local Ethics Committee of Omsk State Medical University. Written informed consent was obtained from all patients.
Bacterial culture and antibiotic susceptibility testing: The cultivation of M. tuberculosis and the testing of drug susceptibility of isolates to 10 primary and secondary anti-TB drugs were carried out over four weeks using the method of absolute concentrations5 in Lowenstein-Jensen (L-J) medium (Merck, USA).
DNA extraction: DNA samples were extracted from pure cultures of M. tuberculosis. The inactivated culture was centrifuged for three min at 3000×g, then the supernatant was removed and 500 μl of solution 1 (2 mM borate buffer pH 9.5, 1% 2-methoxyethanol) was added to the residue, vortexed and heated for 10 min at 95°C. This was centrifuged for another three minutes at 3000×g, and then, the supernatant containing the DNA was transferred to a clean tube and used as a template for the polymerase chain reaction (PCR)7.
Fifteen loci mycobacterial interspersed repetitive units (MIRU)-VNTR typing: MIRU-VNTR typing by amplifying 15 loci (MIRU2, MIRU4, MIRU10, MIRU16, MIRU20, MIRU23, MIRU24, MIRU26, MIRU27, MIRU31, MIRU39, MIRU40, ETRA, ETRB and ETRC) was performed as previously described with some modifications58. The oligonucleotide primer sequences are given in Table I. For each reaction, DNA from M. tuberculosis H37Rv was used as a positive control, and sterile water was used as a negative control. PCR products were electrophoretically separated on 6 per cent polyacrylamide gel, using a 100 bp DNA ladder as a size marker. The database ‘MIRU-VNTRplus’ (http://miru-vntrplus.org) was used to determine the genetic lineage of each isolate9.
Identification of mutations associated with the development of resistance to isoniazid (INH) and rifampicin (RIF): The identification of mutations of katG315 and rpoB531 was performed as previously described using PCR-restriction fragment length polymorphism (RFLP) analysis10. Primers were designed for the amplification of fragments containing katG315 (MSPA and MSPB) and rpoB531 (MUT531U and MUT531R) (Table I).
Statistical analysis: The data were analysed using STATISTICA 6.0 (StatSoft Inc., USA). MIRU-VNTRplus resource (http://www.miru-vntrplus.org/MIRU/index.faces) was used for building an Unweighted Pair Group Method with Arithmetic Mean dendrogram of MIRU-VNTR digital profiles treated as categorical variables. The Hunter-Gaston Discrimination Index (HGDI) was used as an estimate of the allelic diversity for each genotype in the 15 combined loci11, and was calculated12 using the ‘Discriminatory power calculator’ (http://insilico.ehu.eus/).
Results
One hundred clinical isolates taken from patients (85 men and 15 women) with pulmonary TB were included in the study. The ratio of men to women was 5.66:1 (85 vs. 15%), the mean age of patients was 35.6±2.37 years. Fifty patients were HIV-positive. The clinical TB forms were infiltrative (70.0%), disseminated (13.0%) and fibrous-cavernous (12.0%) TB. Primary TB was detected in 82 patients; the others had a chronic form of TB with reinfection and reactivation.
VNTR typing: Thirty six different VNTR genotypes (Figure) were found among the 100 isolates. Twenty six isolates (26%) were unique, and the remaining 74 were grouped into 10 clusters. HGDI was 0.888 and the clustering index was 74.0 per cent. Seventy two isolates belonged to the Beijing family and 61 (85.0%) were grouped into five clusters each containing 2 to 23 isolates. Two major clusters contained 23 isolates and had an allelic profile of 223325173533424 and 223325193533424, respectively.
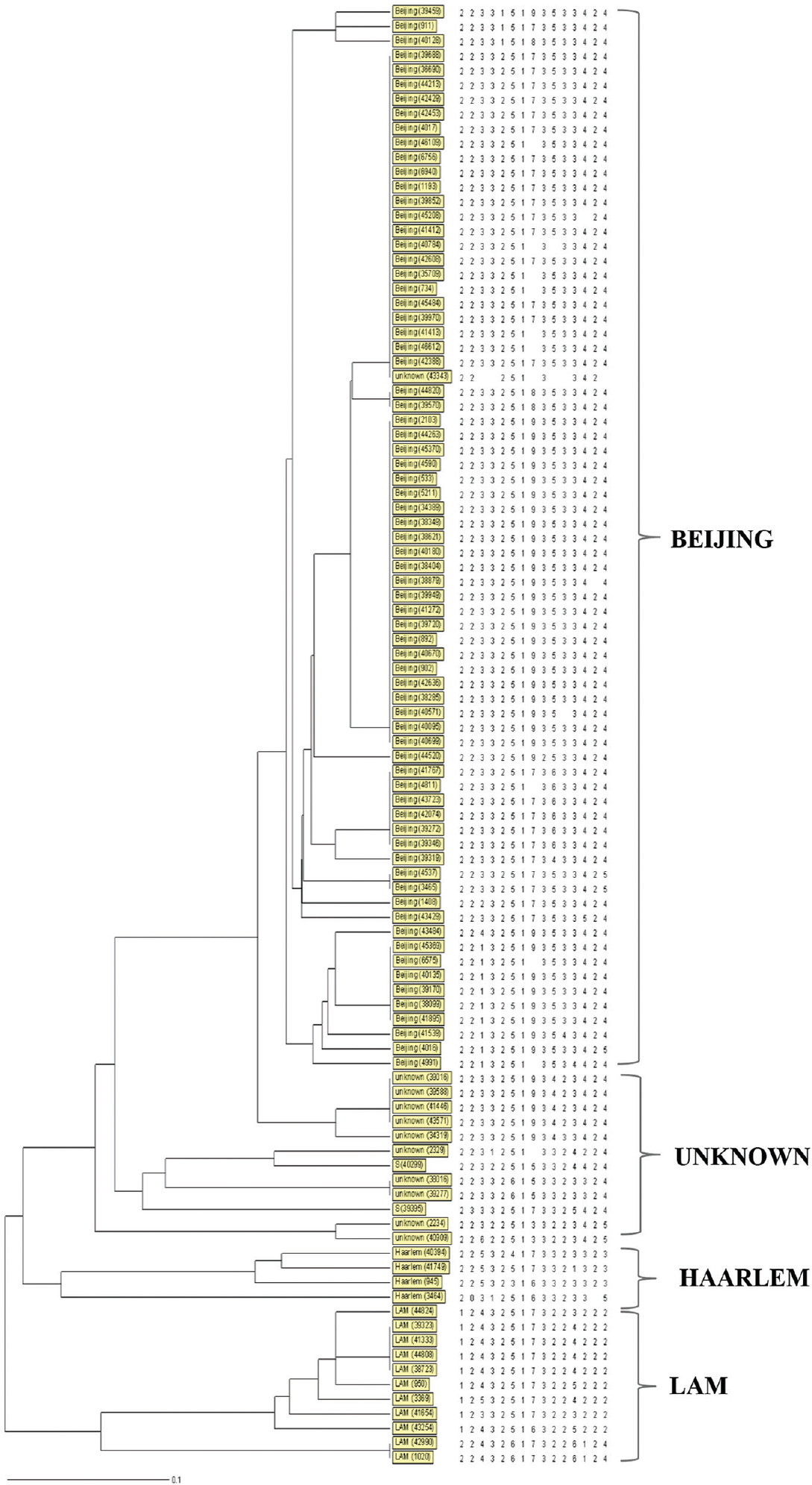
- Dendrogram deduced from clustering analysis of 100 isolates. By using the 15 loci MIRU-VNTR typing (MIRU2, MIRU4, MIRU10, MIRU16, MIRU20, MIRU23, MIRU24, MIRU26, MIRU27, MIRU31, MIRU39, MIRU40, ETRA, ETRB, ETRC) revealed that 100 isolates were categorized into 36 genotypes and 10 clusters, 72 per cent (72/100) isolates belonged to Beijing family.
Other genetic families were also detected: LAM (Latin American Mediterranean) in 11 isolates (11.0%), Haarlem (4.0%) and S family (2.0%).
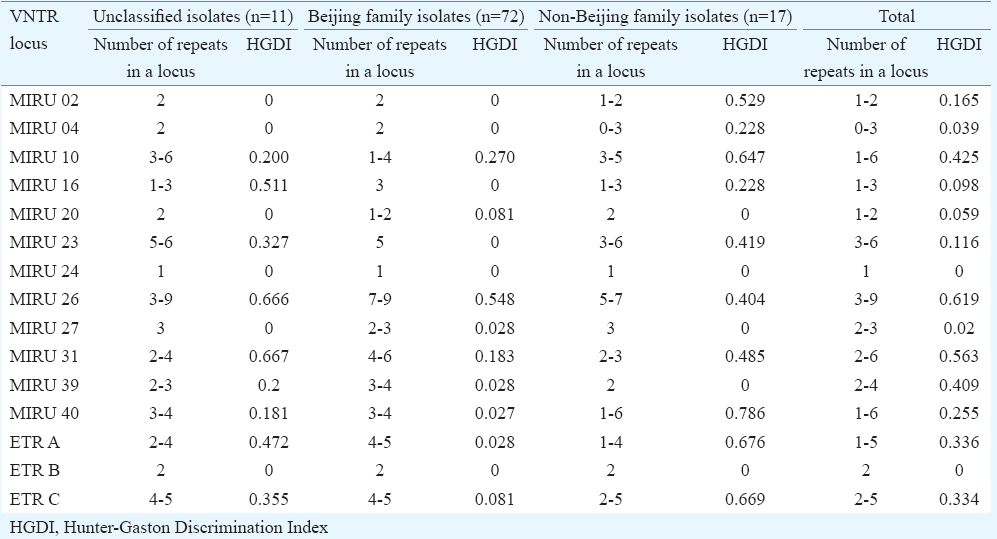
The genetic family of 11 isolates could not be determined (labelled as unknown). Six VNTR profiles were found in these non-classified isolates. Four of these isolates were unique, and the remaining seven were grouped into three clusters. Allelic polymorphism analysis of each MIRU-VNTR locus demonstrated variability in the number of tandem repeats (Table II). The number of alleles detected per locus ranged from one to nine. MIRU16, MIRU26, MIRU31 loci showed the highest level of polymorphism in the unclassified isolates. Among the Beijing isolates, MIRU26 (HGDI=0.548) was the most polymorphic locus, while six loci were monomorphic, having the same allelic profile (MIRU02, MIRU04, MIRU16, MIRU23, MIRU24 and ETRB). The HDGI of the rest of the loci ranged from 0.027 to 0.27. In the non-Beijing isolates (n=17), the MIRU02, MIRU10, MIRU40, ETRA and ETRC loci were polymorphic (Table II).
Only 16 per cent of isolates were susceptible to anti-TB drugs (Table III). The rest were resistant to the first-line drugs in different combinations, such as INH (83%), RIF (77%), streptomycin (72%) and ethambutol (13%), and to the second-line drugs, including ethionamide (30%), ofloxacin (21%), kanamycin (12%), capreomycin (9%), para-aminosalicylic acid (8%) and cycloserine (2%).
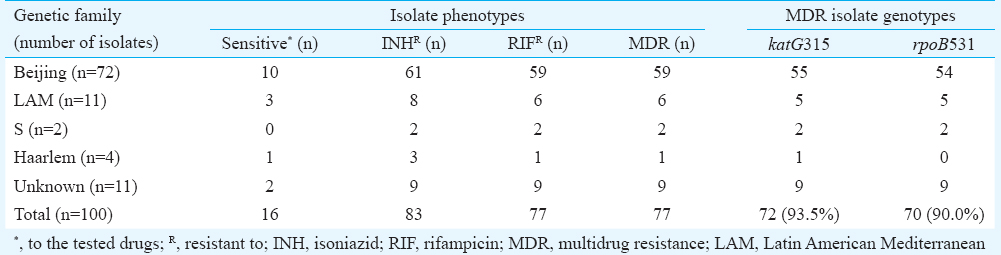
Seventy seven per cent of multiple drug-resistant TB isolates were resistant to at least INH and RIF. Of these, 12 (15.6%) were resistant to all first-line drugs, including streptomycin and ethambutol.
Of the 77 drug-resistant isolates, 59 (76.6%) belonged to the Beijing genotype and 18 to other genetic families. The level of multidrug resistance among Beijing and non-Beijing genotypes did not differ significantly.
Discussion
Epidemiological studies are essential in TB surveillance, along with effective monitoring and preventive measures13. Understanding the distribution of the various M. tuberculosis genotypes allows the impact of genetic polymorphism on the course of the disease deciphered, the epidemiologically significant genotypes to be identified, the infection history to be deduced and its development to be predicted14.
Previous studies have shown a close relationship between the genetic diversity of M. tuberculosis and different geographical regions. The prevalence of the Beijing genotype in different regions in China ranged from 55.3 to 91.5 per cent1516. The Beijing genotype was recorded in 20.3 per cent cases in the north India (Varanasi)13. The Beijing genotype has major epidemiological and clinical value in Russia. The prevalence of the Beijing genotype ranged from 27.0 to 66.8 per cent of cases in different regions of Western and Eastern Siberia (Novosibirsk region, Republic of Buryatia, Tomsk region)101417.
The LAM family was represented by 11 isolates with different allelic profiles. In terms of frequency of occurrence LAM ranks in second place in most regions of Russia (12 to 16%)10181920. The LAM prevalence rate in all studied regions within Russia and the former Soviet Union amounted to 29.4 per cent in Karelia, 25.5 per cent in Pskov, 13.3 per cent in Kaliningrad, 28.6 per cent in Tuva, 19.5 per cent in Kazakhstan and 41.8 per cent in Belarus21.
The LAM family strains in Russia are mainly represented by RD115/LAM-RUS sublineage that was suggested endemic for Northern Eurasia21. Overall, the LAM prevalence is globally variable from as high as 60 per cent in some countries of South America such as Brazil to the negligible one per cent in East Asia e.g., in Tibet, China and Vietnam2223. Based on phylogeographic analysis of the global dataset and robust molecular markers, Mokrousov et al22 suggested that LAM family originated in the Western Mediterranean region although its dating remains unknown and its dispersal was determined by large-scale human migrations flaws.
The S family isolates occurred in 2 per cent of cases; in the Novosibirsk region the prevalence of this family was 7 per cent, but it reached 90 per cent in Yakutia among the indigenous people, where it was suggested to be endemic1024. S family is one of the M. tuberculosis genetic families named so because first identified in Sicily and later in Sardinia2526.
The Haarlem family was found in 4 per cent of cases, while in South America this family was predominant among drug-resistant M. tuberculosis (43.6%)27.
The MDR subsample in this study included 93.5 per cent isolates with katG315 mutation and 90 per cent of isolates with rpoB531 mutation. The predominance of these major mutations had previously been shown in the Novosibirsk oblast510. Under the influence of selective pressure their accumulation takes place, and phenotypically, there is an increase of the proportion of MDR strains in the population of M. tuberculosis. The presence of insignificant number of mutational events indicates that these are an epidemic type of M. tuberculosis that is actively spreading in the Omsk region. Such isolates can be epidemiologically linked to recent contamination from the public infection reservoir, despite the lack of direct contact with isolated foci1124.
Considerable clustering and the presence of two large clusters belonging to the Beijing family with numerous branches in the dendrogram defined the dissemination and active circulation of this genotype in the Omsk region. Perhaps, the lack of a clear epidemiological link between patients in clusters confirms the active dissemination of drug-resistant M. tuberculosis isolates among the population in the Omsk region.
This study had some limitations. One is the high proportion of M. tuberculosis drug-resistant isolates compared with population-based studies. Further, we were not able to use 15-loci MIRU-VNTR typing to determine the genetic family of 11 isolates. This can be attributed to the lack of a sufficient number of MIRU loci and the appearance of new genetic variants of M. tuberculosis in the Omsk region.
Larger studies with representative sampling need to elucidate the actual status and role of these genotypes in the dissemination and transmission of TB in the southern part of West Siberia. This is a baseline study of the M. tuberculosis population structure in Omsk region, and may serve as an impetus for future molecular epidemiological studies in the southern part of West Siberia, and will help to estimate genetic diversity of the global tubercle bacilli.
In conclusion, the use of 15-locus VNTR typing revealed the circulation of different genetic families, including Beijing, LAM, S and Haarlem. The predominance of Beijing genotype was found among isolates with multidrug resistance. The most common mutations among the MDR isolates were katG315 and rpoB531. The data obtained are crucial for the selection and evaluation of the effective preventive measures to reduce the spread of MDR strains in the Omsk region.
Acknowledgment
Authors acknowledge the staff of the Bacteriological Laboratory of the TB Hospital for their support, and thank all doctors, bacteriologists, nurses and patients who actively participated in this study. This study was financially supported by the FGBOU OSMU, Russian Federation (no. VG-21-2014).
Conflicts of Interest: None.
References
- Available from: http://www.who.int/mediacentre/factsheets/fs104/en
- World Health Organization. Global Tuberculosis Report 2015. Available from: http://www.who.int/tb/publications/global_report/en
- [Google Scholar]
- Epidemical impact of tuberculosis mycobacteria circulating in Omsk Region. Tuberk Bolezni Legkih. 2015;6:12-4.
- [Google Scholar]
- Changes in tuberculosis epidemiological rates in Omsk region. Tuberk Bolezni Legkih. 2015;5:139-40.
- [Google Scholar]
- Characterization of extensively drug-resistant Mycobacterium tuberculosis isolates circulating in Siberia. BMC Infect Dis. 2014;14:478.
- [Google Scholar]
- Mycobacterium tuberculosis phylogeography in the context of human migration and pathogen's pathobiology: Insights from Beijing and Ural families. Tuberculosis (Edinb). 2015;95(Suppl 1):S167-76.
- [Google Scholar]
- Molecular epidemiology of tuberculosis and other mycobacterial infections: Main methodologies and achievements. J Intern Med. 2001;249:1-26.
- [Google Scholar]
- Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol. 2006;44:4498-510.
- [Google Scholar]
- Evaluation and strategy for use of MIRU-VNTRplus, a multifunctional database for online analysis of genotyping data and phylogenetic identification of Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2008;46:2692-9.
- [Google Scholar]
- Highest prevalence of the Mycobacterium tuberculosis Beijing genotype isolates in patients newly diagnosed with tuberculosis in the Novosibirsk oblast, Russian Federation. J Med Microbiol. 2011;60(Pt 7):1003-9.
- [Google Scholar]
- Numerical index of the discriminatory ability of typing systems: An application of Simpson's index of diversity. J Clin Microbiol. 1988;26:2465-6.
- [Google Scholar]
- In silico analysis of complete bacterial genomes: PCR, AFLP-PCR and endonuclease restriction. Bioinformatics. 2004;20:798-9.
- [Google Scholar]
- A study of Mycobacterium tuberculosis genotypic diversity & drug resistance mutations in Varanasi, North India. Indian J Med Res. 2014;139:892-902.
- [Google Scholar]
- Investigation phylogenetic relationships major genotypes Mycobacterium tuberculosis by MIRU-VNTR-24 genotyping. Bull East Sib Sci Cent Sib Branch Russ Acad Med Sci. 2013;2:144-7.
- [Google Scholar]
- Molecular typing of Mycobacterium tuberculosis isolates circulating in Jiangsu province, China. BMC Infect Dis. 2011;11:288.
- [Google Scholar]
- Genotypes and drug susceptibility of Mycobacterium tuberculosis Isolates in Shihezi, Xinjiang Province, China. BMC Res Notes. 2012;5:309.
- [Google Scholar]
- Epidemiological and immunopatological features of Beijing tuberculosis from Tomsk region. Epidemiol Vakcinoprofil. 2011;3:4-10.
- [Google Scholar]
- Genotype LAM of Mycobacterium tuberculosis in Buryatia. Siberian Medical Journal (Irkutsk). 2013;6:140-2.
- [Google Scholar]
- Identification of ubiquitous and endemic Mycobacterium tuberculosis genotypes in the Republic of Buryatia. Mol Gen Mikrobiol Virusol. 2014;2:12-6.
- [Google Scholar]
- Mycobacterium tuberculosis population in Northwestern Russia: An update from Russian-EU/Latvian border region. PLoS One. 2012;7:e41318.
- [Google Scholar]
- Mycobacterium tuberculosis Latin American-Mediterranean family and its sublineages in the light of robust evolutionary markers. J Bacteriol. 2014;196:1833-41.
- [Google Scholar]
- Latin-American-Mediterranean lineage of Mycobacterium tuberculosis: Human traces across pathogen's phylogeography. Mol Phylogenet Evol. 2016;99:133-43.
- [Google Scholar]
- Stranger in a strange land: Ibero-American strain of Mycobacterium tuberculosis in Tibet, China. Infect Genet Evol. 2014;26:323-6.
- [Google Scholar]
- Molecular epidemiological features of M.tuberculosis family S in Sakha (Yakutia) Siberian Medical Journal (Irkutsk). 2014;6:109-11.
- [Google Scholar]
- Genetic diversity of Mycobacterium tuberculosis in Sicily based on spoligotyping and variable number of tandem DNA repeats and comparison with a spoligotyping database for population-based analysis. J Clin Microbiol. 2001;39:1559-65.
- [Google Scholar]
- Mycobacterium tuberculosis molecular evolution in western Mediterranean Islands of Sicily and Sardinia. Infect Genet Evol. 2005;5:145-56.
- [Google Scholar]
- Characterization of the genetic diversity of extensively-drug resistant Mycobacterium tuberculosis clinical isolates from pulmonary tuberculosis patients in Peru. PLoS One. 2014;9:e112789.
- [Google Scholar]






