Translate this page into:
Safety of yellow fever vaccine in Indian travellers: A prospective observational study
*For correspondence: ptiwari@niper.ac.in
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Yellow fever (YF), a disease caused by the Flavivirus, is endemic in geographically limited places (Sub-Saharan Africa and tropical South America)12. According to the World Health Organization estimates, globally, YF infection causes an estimated 30,000 deaths and 200,000 cases of disease annually3. The symptoms of the disease vary from mild, febrile illness to severe with multi-organ failure, jaundice and haemorrhage4. YF vaccination, in use for over 70 years, is considered to be safe and effective. The side effects of YF vaccine vary from mild fever to YF vaccine-associated viscerotropic disease and neurological disease5. The increase in the number of travellers from India to YF endemic regions for various reasons has led to a rise in the demand for vaccination in India. Tiwari et al6 in their pilot study on a limited number of travellers have confirmed the safety and recommended similar study in a larger sample to report the rare adverse drug reactions (ADR). This study was carried out to determine the safety and tolerability of YF vaccine in healthy Indian travellers.
This prospective observational study was conducted from March 2012 to March 2013, at a travel health outpatient setting in Punjab, India. The study was approved by the Human Ethics Committee of the National Institute of Pharmaceutical Education and Research (NIPER), Mohali, India. Travellers vaccinated for YF and over nine months of age were included in the study on a consecutive basis. The YF vaccine Stamaril® (Sanofi-Pasteur, France) consisting of a live attenuated strain of YF virus (17 D-20 strain) was used in the study. Briefly, the travellers were followed for the occurrence of fever, malaise and myalgia, headache, nausea, vomiting, abdominal pain, diarrhoea, urticaria, local reaction and any other serious adverse event, on days 7 and 14, telephonically, post vaccination6. According to the Centers for Disease Control and Prevention (CDC), the incubation period of the AE due to YF varied from 1 to 10 days7.
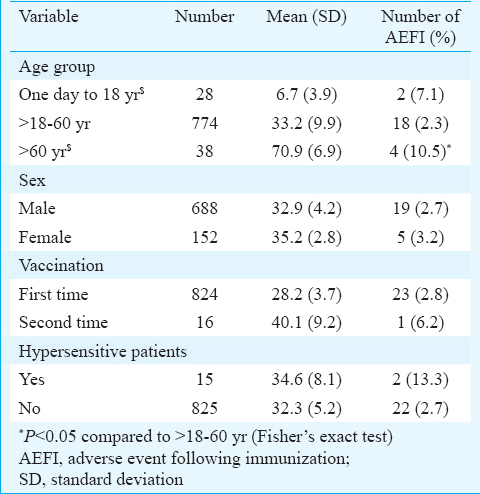
Adverse events were further classified as mild, moderate and severe. A sample size of 778 was required, considering expected incidence rate of ADRs of 5 in 1000, the statistical power of 90 per cent and anticipated occurrence of adverse event following immunization (AEFI) as 2, from existing literature8. Travellers were enrolled from a travel health clinic in Mohali. A total of 849 vaccinated travellers were included in the study. Eight hundred and forty travellers were successfully followed up for the occurrence of AEFI. The mean age of vaccinated travellers was 34.2±4.6 yr. Of all, only 152 travellers were female (the male to female ratio was 1:0.22). The difference in age of male and female was not significant. Of all vaccinated travellers, 16 were vaccinated second time; only 15 travellers were found to be hypersensitive toward a medicine or food item.
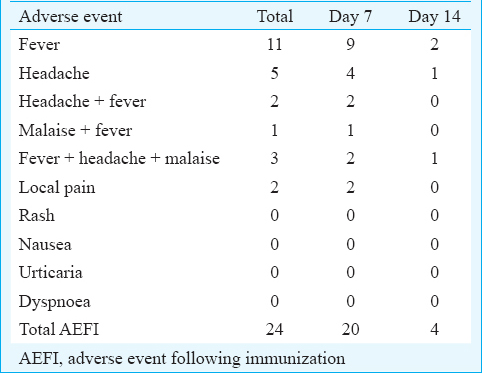
The vaccinated travellers were followed on days 7 and 14 after vaccination, telephonically, for the occurrence of any AEFI. Twenty AEFI were reported on day 7 follow up while only four were reported on day 14 post-vaccination (Table I). Acute hypersensitivity reaction was not reported following vaccination. Mild AEFI was observed in 24 travellers. No moderate and major AEFI were observed. Fever was found to be the most common AEFI and occurred in 11 travellers followed by a headache in five (Table II). The local pain was observed in only two travellers. Fever along with headache and malaise was reported in six. No case of rash, nausea, urticaria and dyspnoea was reported among travellers.
IBM SPSS statistics package v. 20.0 (IBM SPSS Corp, Armonk, NY, USA) was used for data analysis. Variables were presented as numbers, percentages and mean with standard deviation. Fisher's exact test was used to assess the relationship between the variables of occurrence of AEFI. Independent sample t test was used to assess the difference in age groups.
Fisher's exact test revealed that the occurrence of mild AEFI in males (AEFI = 19 of 688) was not significantly different from that in females (AEFI = 5 of 152). Most of the vaccinated travellers belonged to age group of >18-60 yr (774 travellers) followed by geriatric and paediatric (38 and 28 vaccinated travellers, respectively). The occurrence of mild AEFI was found significantly higher in the geriatric age group when compared to adults (P<0.05). The occurrence of AEFI was not significantly different with respect to traveller's allergic history and traveller's previous YF vaccination history (Table II).
Only those travelling to endemic areas need to be vaccinated9. According to the revised recommendation of advisory committee on immunization practice and CDC, only one vaccination provides lifelong immunity in travellers with different medical conditions, geographical areas and of all age group1. The earlier recommendations demanded a re-vaccination if the traveller intends to move in the endemic region again2.
In this study 79 per cent (19/24) of the AEFIs were noted in male travellers. Biscayart et al10 have reported AEFI in 77 per cent of the males. The predominance of male travellers in the present study was consistent with a previous report11. This may be due to a higher number of male travellers. Only 28 and 38 of the travellers belonged to paediatric and geriatric age category, respectively. Business was the primary reason for travel and accounted for a higher number of adult travellers.
In this study, fever and headache were reported in 1.3 and 0.6 per cent of the travellers, respectively. These were lower than that reported earlier812. According to Durbin et al8, fever and headache were found to occur in three and 2.8 per cent travellers, respectively.
Mild AEFI were reported in 2.9 per cent of the vaccinated travellers, who did not require any specific treatment. The extent of AEFI was found to be smaller than the earlier reports812. Durbin et al8 reported mild side effects in four per cent of the 2326 YF vaccinated travellers through a TeleWatch system. According to Camacho et al12, the excess risk of any systemic adverse events ranged from 3.5 to 7.4 per cent across the vaccinated travellers. In the present study, no case of acute hypersensitivity reaction was noted.
In conclusion our results suggested that YF vaccination led to only mild side effects; and, none of these required medical care. On this basis, YF vaccine appears to be safe and well tolerated in healthy Indian travellers of all ages and gender.
The study had some limitations. First, the study was not powered to capture the data on the extremely rare AEFI like the neurotropic or viscerotropic disease. Second, it did not have the matched control group. Third, the long-term follow up of the travellers was not possible due to an early departure to the destination country.
Acknowledgment
The authors acknowledge the National Institute of Pharmaceutical Education and Research, SAS. Nagar, Punjab, India, for providing the resources to carry out the study.
Conflicts of Interest: None.
References
- Centers for Disease Control and Prevention (CDC).Yellow fever vaccine booster doses: recommendations of the Advisory Committee on Immunization Practices, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:647-50.
- [Google Scholar]
- Yellow fever vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2002. MMWR Recomm Rep. 2002;51:1-11.
- [Google Scholar]
- World Health Organization. Division of epidemiological surveillance and health situation trend assessment. In: Global health situation and projections – estimates. Geneva, Switzerland: World Health Organization; 1992.
- [Google Scholar]
- Yellow fever vaccine. In: Plotkin S, Orenstein WA, Offit PA, eds. Vaccines (5th ed). Philadelphia: WB Saunders; 2008. p. :959-1055.
- [Google Scholar]
- Safety profile of the yellow fever vaccine Stamaril®: a 17-year review. Expert Rev Vaccines. 2013;12:1351-68.
- [Google Scholar]
- Evaluation of safety profile of yellow fever vaccine in healthy Indian travellers: a prospective observational study. J Pharm Care Health Syst. 2015;2:134.
- [Google Scholar]
- Yellow Fever History, Epidemiology, and Vaccination Information. Mild Adverse Events: CDC. Available from: http://www.cdc.gov/travel-training/local/History EpidemiologyandVaccination/page27367.html
- Monitoring adverse events following yellow fever vaccination using an integrated telephone and Internet-based system. Vaccine. 2009;27:6143-7.
- [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1-27.
- [Google Scholar]
- Yellow fever vaccine-associated adverse events following extensive immunization in Argentina. Vaccine. 2014;32:1266-72.
- [Google Scholar]
- Review of the risks and benefits of yellow fever vaccination including some new analyses. Expert Rev Vaccines. 2012;11:427-48.
- [Google Scholar]
- Reactogenicity of yellow fever vaccines in a randomized, placebo-controlled trial. Rev Saude Publica. 2005;39:413-20.
- [Google Scholar]





