Translate this page into:
Preventive role of carvedilol in adriamycin-induced cardiomyopathy
Reprint requests: Dr Rajesh Jhorawat, 136, Swaroop Vihar, Jagatpura, Jaipur 302 025, Rajasthan, India e-mail: jhorawat2000@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Adriamycin though considered as an effective anticancer drug, leads to irreversible cardiomyopathy (CMP) and congestive heart failure (CHF). The aim of this study was to determine the protective effect of carvedilol in adriamycin (ADR)-induced cardiomyopathy (CMP) in cancer patients.
Methods:
Patients with lymphoreticular malignancy in whom ADR therapy was planned were randomized into two groups: carvedilol and control. Twenty seven patients each were enrolled in carvedilol and control groups. In the carvedilol group, 12.5 mg once daily oral carvedilol was given during six months. The patients were evaluated by echocardiography before and after chemotherapy. Left ventricular ejection fraction (EF) and systolic and diastolic diameters were calculated.
Results:
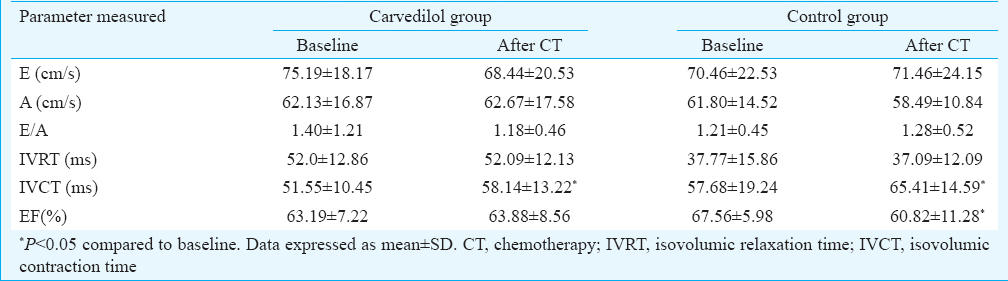
At six months of follow up, six patients in the carvedilol group and five in the control group had died. The mean EF (63.19 vs. 63.88%) and fraction shortening (FS) (34 vs. 34.6) of the carvedilol group were similar at follow up, but in the control group, the mean EF (67.27 vs. 60.82%, P=0.003) and FS (38.48 vs. 34.6, P<0.05) at control echocardiography were significantly lower. In carvedilol group, both systolic and diastolic diameters were not changed, but in control group, systolic diameters were significantly increased compared with basal measures (left ventricular end systolic diameter = 28.26±5.50 mm vs. 31.25± 6.50 mm; P< 0.05).
Interpretation & conclusions:
Prophylactic use of carvedilol in patients receiving anthracycline protected systolic functions of the left ventricle. Carvedilol can be a potential drug which can ameliorate ADR-induced CMP.
Keywords
Adriamycin
cardiomyopathy
carvedilol
chemotherapy
ejection fraction
fraction shortening
Anthracyclines (ANTs) rank amongst the most effective anticancer drugs ever developed1. However, their popularity was hampered by serious problems such as the development of resistance in tumour cells or toxicity in healthy tissues, most notably in the form of irreversible cardiomyopathy (CMP) and congestive heart failure (CHF). In both children and adults, risk of cardiotoxicity increases with the total dose of adriamycin (ADR). A cumulative dose of >550 mg/m2 has a five-time higher risk of cardiotoxicity than a lower cumulative dose2.
The postulated mechanisms of doxorubicin CMP include formation of reactive oxygen species (ROS) and increased oxidative stress through multiple pathways3. Enzymatic protection of cells against oxygen radicals such as superoxides and peroxides consists of glutathione peroxidase, superoxide dismutase and catalase45. In animal models, it has been shown that cardiac concentrations of these protective enzymes are far lower than those in other organs56. This may result in the impairment of cardiac contractility and development of CMP after ANT use7.
Different chemical agents such as dexrazoxane, N-acetylcysteine, vitamin E, A and C, amifostine, carvedilol, coenzyme Q10, carnitine, probucol, carotenoids, selenium and glutathione have been shown to prevent ANT-induced CMP8910. Carvedilol, a new generation β-blocker which blocks β-1, β-2 and α-1 adrenoceptors has been used for dilated CMP, and has potent antioxidant and antiapoptotic properties11. In animal and human studies it has been shown that carvedilol prevented the development of CMP, free radical release and apoptosis in cardiomyocytes due to chemotherapeutics1213. Information concerning prophylactic carvedilol use in preventing ANT-induced CMP in Indian patients is lacking. Therefore, we designed this study to establish the protective effect of carvedilol in ADR induced CMP in cancer patients.
Material & Methods
The study was done at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. Patients diagnosed with lymphoreticular malignancy and planned for chemotherapy (CT) with regimen containing ANT (ADR) between January 2008 and February 2009 were enrolled from Adult Hematology Clinic. A total of 54 consecutive patients aged ≥18 yr were enrolled in the study according to inclusion criteria. Those who were found not fit according to exclusion criteria were excluded and the remaining patients were randomised to either group according to random table. The exclusion criteria included earlier CT or thoracic radiotherapy, coronary arterial disease or established dilated or restrictive CMP, moderate-to-severe valvular dysfunction or pericardial effusion, diabetes mellitus, renal dysfunction and thyroid disorder. Any contraindication to carvedilol, for example, hypersensitivity, bronchial asthma, second- and third-degree atrioventricular block, sick sinus syndrome, severe hepatic impairment and intake of other drugs that affected cardiac functions, for example, angiotensin converting enzyme (ACE)-inhibitor, angiotensin receptor blockers, diuretics or β-blockers, statins and antioxidants were excluded from the study.
All patients received CT at a mean of every 3-4 wk. Patients were randomly divided into two groups (carvedilol vs. control) on the basis of a random table. In the carvedilol group, 12.5 mg once daily oral carvedilol was started before CT and maintained for six months during CT. The primary end point in this study was systolic functions and death of the patients. This study was approved by the institutional Ethics Committee, and written consent was taken from all patients.
Echocardiography: All patients were evaluated by two-dimensional (2D), pulsed-wave Doppler and tissue Doppler imaging echocardiography. Echocardiographic examinations were performed using ultrasound [Acuson Sequoia (512)] equipped with a 2.5 to 4.0 MHz (AcusonV4c) transducer (Siemens, Germany). Left ventricular systolic and diastolic diameters and ejection fraction (EF) were calculated. In transmitral pulsed Doppler examination, the peak velocities of early (E) and late diastolic flow (A), the E/A ratio, isovolumic relaxation time and isovolumic contraction time were measured. One cardiologist who was blinded to the patients’ clinical and laboratory data interpreted each echocardiographic examination.
Cardiologic assessment: In all patients, resting electrocardiogram and echocardiography were done before starting CT as baseline and after completing CT or in between if the patient became symptomatic during CT. Systolic dysfunction was defined as EF <50 per cent. Diastolic function was evaluated according to changes in mitral inflow parameters.
The Cardiac Review and Evaluation Committee (CREC)14 has established criteria for the diagnosis of CT-related cardiac dysfunction (CRCD) as: (i) CMP characterized by a decrease in cardiac left ventricular EF (LVEF), either global or more severe in the septum; (ii) symptoms of HF; (iii) associated signs of HF including but not limited to S3 gallop, tachycardia or both; and (iv) decline in LVEF of at least five per cent to less than 55 per cent with accompanying signs or symptoms of HF, or a decline in LVEF of at least 10 per cent to below 55 per cent without accompanying signs or symptoms. The presence of any one of the four criteria was considered sufficient to confirm a diagnosis of CRCD14.
Statistical analysis: A paired t test was used to investigate the time-dependent variables and Student's t test to compare two groups. Comparison of LV dysfunction between two groups was done using Chi-square test. SPSS 13 software (Chicago, IL, USA) was used for statistical analysis.
Results
A total of 54 newly diagnosed patients with haematological malignancy, who received ADR in their CT regimen were studied. Baseline characteristics of the patients are shown in Table I. By the end of follow up, six patients in carvedilol group and five patients in the control group died. Mortality rate between the two groups was not significant. During follow up, three patients in control group and only one patient in carvedilol group had EF <50 per cent who were treated. However, LV dysfunction defined as a decrease in 10 per cent EF from baseline was 14.3 and 40.9 per cent in carvedilol and control group, respectively (P=0.053). There was no significant change in EF in carvedilol group but a significant decrease in EF was seen in control group (P=0.003) (Table II). Fractional shortening in carvedilol group did not change, but in control group, it decreased from 38.48 to 34.6 (P<0.05).
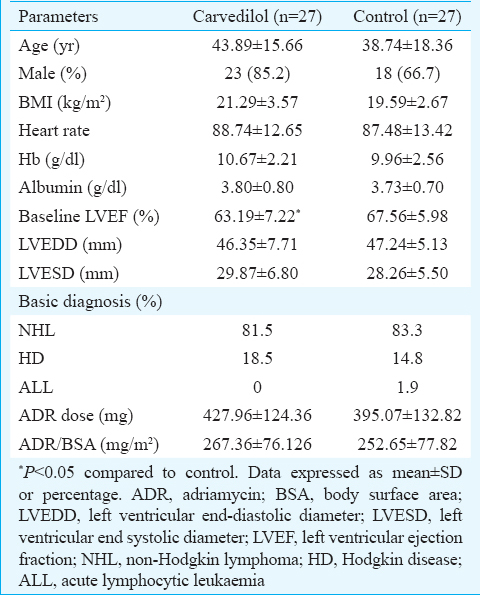
Although there was no significant change in both systolic and diastolic diameters of LV in the carvedilol group [left ventricular end systolic diameter (LVESD) = 29.89±6.80 vs. 30.30±6.04 mm; and left ventricular end diastolic diameter (LVEDD) = 46.35±7.71 vs. 47.95±5.28 mm], in control group, there was a significant change in systolic diameters (LVESD = 28.26±5.50 vs. 31.25±6.50 mm; P<0.05) with no significant change in the diastolic diameters of LV (LVEDD = 47.24±5.13 vs. 48.50±5.75 mm), indicating subclinical systolic dysfunction in the control group patients.
Doppler study was done for both the groups (Table II). There was no significant change in the diastolic parameter of LV of the two groups; however, change in isovolumic relaxation time (IVCT) was significant in both the groups (P<0.05) (Table II).
Discussion
Carvedilol is an antioxidant and free radical scavenger, which inhibits the production of oxidized low-density lipoproteins and the generation of oxygen radicals by neutrophils15. The mean EF and fraction shortening (FS) after CT in the carvedilol group were similar to baseline EF and FS but significantly decreased in the control group. Systolic function was better preserved in the carvedilol group compared to the control group.
In our study, patients with LV systolic abnormalities had mild or moderately increased LV diameters, but in patients receiving carvedilol, LV diameters did not increase. LV diastolic function did not change in the control group. A previous study16 showed that LV diastolic functions might also be impaired in patients receiving CT; however, in our study, LV diastolic diameter increased in the control group, but the changes were not significant. Cardiac damage has been shown at cumulative doses as low as 200 mg/m2, well below levels assumed to induce injury17.
Six patients in carvedilol group and five patients in control groups died. Total mortality was higher in our study compared with an earlier similar human study7. Carvedilol has a potent antioxidant activity that inhibits oxygen radical formation. These antioxidant effects have been demonstrated in a variety of in vitro and in vivo experimental models and originate from the unique carbazole moiety of carvedilol1417. Carvedilol is approximately 10-fold more potent than vitamin E as an antioxidant. Some of the metabolites of carvedilol are more potent antioxidants and approximately 1000-fold more potent than vitamin E18. It is possible that one or more of these metabolites may contribute to the antioxidant activity of carvedilol.
Based on its molecular mechanisms of action, carvedilol seems to have additional properties other than as a β-blocker which is not shared by other members of its group. Carvedilol is superior to propranolol in the prevention of the mitochondrial dysfunction, prevents hydroxyl radical-induced cardiac contractile dysfunction, and prevents ANT-induced apoptosis121920. These data suggest that carvedilol is superior to other β-blockers for preventing ANT-induced CMP owing to its antioxidant and antiapoptotic properties.
Various doses of carvedilol have been used in earlier studies. The dose used in the Multicenter Oral Carvedilol HF Assessment trial21 was 12.5 to 50 mg. In our study carvedilol was used at 12.5 mg dose once daily because the antioxidant properties of carvedilol have been documented at a low dose and a single dose facilitates patient compliance with therapy. However, further clinical studies are needed to find the most appropriate dose.
Bosch et al22 have shown that carvedilol with enalapril can prevent left ventricle systolic dysfunction due to ANT-induced cardiotoxicity in patients with malignant haemopathies treated with intensive CT. The role of prophylactic β-blocker and angiotensin receptor blocker therapy is also under active investigation in patients undergoing epirubicin therapy in the PRevention of cArdiac Dysfunction during Adjuvant (PRADA) breast cancer therapy study23. A systematic review and meta-analysis on prophylactic pharmacologic agents in the prevention of chemotherapy-induced LV dysfunction showed that dexrazoxane, β-blocker, statin or angiotensin antagonist had similar efficacy for reducing cardiotoxicity24.
The main limiting factor of our study was enrolment of a limited number of patients. No benefit in the mortality in carvedilol group may be due to the limited number of patients or may be due to low ADR dose used in both the groups. Further, cardiac evaluation by echocardiography is operator dependent. We did not compare our echocardiography finding with other imaging such as strain/3D echo or tissue Doppler studies or multigated-acquisition. This might have affected the results of the study. Dose of ADR used in our study was low. This might explain why significant LV dysfunction did not occur in the control group compared to carvedilol group. The baseline EF was different in the two groups; however, the follow up EF and LV systolic parameters were preserved better in the carvedilol group than the control group. Finally, our study was not placebo controlled.
In conclusion, ADR-induced CMP is an important iatrogenic irreversible complication that needs preventive strategies. Carvedilol is a unique β-blocker which can ameliorate CT-induced CMP. More randomized clinical trials are needed to define the role of carvedilol both in acute and chronic onset CT-induced CMP before considering this drug as a routine prophylactic agent against ADR-induced CMP.
Conflicts of Interest: None.
References
- The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992;19:670-86.
- [Google Scholar]
- Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol. 1997;15:1544-52.
- [Google Scholar]
- Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc. 2014;3:e000665.
- [Google Scholar]
- The role of GSH peroxidase in protecting the membrane of rat liver mitochondria. Biochim Biophys Acta. 1970;223:210-3.
- [Google Scholar]
- Adriamycin stimulated superoxide formation in submitochondrial particles. Chem Biol Interact. 1977;19:265-78.
- [Google Scholar]
- Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980;65:128-35.
- [Google Scholar]
- Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258-62.
- [Google Scholar]
- The protective effect of melatonin on adriamycin-induced acute cardiac injury. Can J Cardiol. 2003;19:535-41.
- [Google Scholar]
- Mice primed with swainsonine are protected against doxorubicin-induced lethality. Cell Mol Biol (Noisy-le-grand). 2003;49:1089-99.
- [Google Scholar]
- Protective effects of erdosteine against doxorubicin-induced cardiomyopathy in rats. J Appl Toxicol. 2003;23:71-4.
- [Google Scholar]
- Carvedilol: molecular and cellular basis for its multifaceted therapeutic potential. Cardiovasc Drug Rev. 2001;19:152-71.
- [Google Scholar]
- Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol. 2004;37:837-46.
- [Google Scholar]
- Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J Card Fail. 2012;18:607-13.
- [Google Scholar]
- Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102:14-25.
- [Google Scholar]
- Carvedilol, a new vasodilating beta adrenoceptor blocker antihypertensive drug, protects endothelial cells from damage initiated by xanthine-xanthine oxidase and neutrophils. Cardiovasc Res. 1994;28:400-6.
- [Google Scholar]
- Carvedilol, a new beta adrenoceptor antagonist and vasodilator antihypertensive drug, inhibits oxygen-radical-mediated lipid peroxidation in swine ventricular membranes. Pharmacol Commun. 1992;1:27-35.
- [Google Scholar]
- Carvedilol, a new beta-adrenoceptor antagonist and vasodilator antihypertensive drug, inhibits superoxide release from human neutrophils. Eur J Pharmacol. 1992;214:277-80.
- [Google Scholar]
- Myocardial protection by the novel vasodilating beta-blocker, carvedilol: potential relevance of anti-oxidant activity. J Hypertens Suppl. 1993;11:S41-8.
- [Google Scholar]
- Advantages in the use of carvedilol versus propranolol for the protection of cardiac mitochondrial function. Rev Port Cardiol. 2004;23:1291-8.
- [Google Scholar]
- Effect of beta-blockers on free radical-induced cardiac contractile dysfunction. Circulation. 1999;100:346-53.
- [Google Scholar]
- Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation. 1996;94:2807-16.
- [Google Scholar]
- Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies. J Am Coll Cardiol. 2013;61:2355-62.
- [Google Scholar]
- Rationale and design of the prevention of cardiac dysfunction during an Adjuvant Breast Cancer Therapy (PRADA) Trial. Cardiology. 2012;123:240-7.
- [Google Scholar]
- Role of cardioprotective therapy for prevention of cardiotoxicity with chemotherapy: a systematic review and meta-analysis. Eur J Cancer. 2013;49:2900-9.
- [Google Scholar]






