Translate this page into:
Use of hydrogen peroxide vapour & plasma irradiation in combination for quick decontamination of closed chambers
Reprint requests: Dr Devendra T. Mourya, Microbial Containment Complex, National Institute of Virology, Sus Road, Pashan, Pune 411 021, Maharashtra, India e-mail: directorniv@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Various conventional methods such as gaseous, vapour and misting systems, fogging, manual spray and wipe techniques employing a number of chemical agents are used for decontamination of enclosed spaces. Among all these methods, use of aerosolized formaldehyde is the most preferred method due to cost-effectiveness and practical aspects. However, being extremely corrosive in nature generating very irritating fumes and difficulty in maintaining a high level of gas concentration, many laboratories prefer the vaporization of hydrogen peroxide (H2O2) as an alternative. We present here the results of using H2O2 vapour in combination with plasma irradiation for quick decontamination of closed chambers.
Methods:
The present study describes a decontamination method, using plasma irradiation in combination with H2O2 (5%). Effect of plasma irradiation and H2O2 on the viability of bacterial spores (Bacillus subtilis), Chikungunya and Kyasanur Forest Disease viruses was assessed.
Results:
Data suggest that with the combination of H2O2 vapour and plasma irradiation, within short time (three minutes), decontamination of surfaces and space volume could be achieved. Although it showed damage of spores present on the strips, it did not show any penetration power.
Interpretation & conclusions:
The results were encouraging, and this method was found to be efficient for achieving surface sterilization in a short time. This application may be useful in laboratories and industries particularly, those working on clean facility concept following good laboratory and manufacturing practices.
Keywords
Biosafety cabinets
formaldehyde
free radicals
hydrogen peroxide
plasma irradiation
Formaldehyde has been used extensively as a fumigant, disinfectant and sterilant. Fumigation with formaldehyde vapour in chambers is the recognized and most commonly used method123 although an alternative system using vaporized hydrogen peroxide (H2O2) is available45. The fumigation with formaldehyde vapour is not a preferred method in hospital settings6 due to highly carcinogenic nature though it is scientifically more promising. Formaldehyde vapour has a weak penetrating ability, and if used in an atmosphere with minute traces of chlorine, it can quickly produce bis(chloromethyl)ether7, which is a known carcinogenic agent. H2O2 vapour is favoured since it exhibits rapid sporicidal activity without pernicious residuum at 20-30°C. Further, the decomposition products of H2O2 have low tissue toxicity24. When working with decontamination of biosafety cabinets and isolators89, development of biofilms on impervious surfaces is another problem encountered. The biofilms sometimes colonize the entire surface of the isolator interior and impervious surfaces of biosafety cabinet to form patches of various thicknesses. These sites prevent the diffusion of dissolved oxygen causing the aerobic microorganisms to become spores. However, it is suggested that H2O2, in combination with ultraviolet (UV rays), can inactivate spores by photodesorption1011. H2O2 is active against a wide range of microorganisms, including bacteria, yeasts, fungi, viruses, prions and spores. A 0.5 per cent accelerated H2O2 demonstrated bactericidal and virucidal activity in one minute and mycobactericidal and fungicidal activity in five minutes1213141516.
Efficacy of H2O2 depends on micro-condensation within the biosafety cabinets, isolators, equipment or laboratory/hospital rooms17. The commercial products contain a larger proportion of water. Water has a higher vapour pressure than H2O2 and vaporizes faster than H2O2 from an aqueous solution. Due to lower molecular weight, water diffuses faster than H2O2 in the vapour site. This is one of the factors that reduce its decontamination capabilities. To overcome some of the practical difficulties of H2O2 vaporization and achieve better decontamination, we used H2O2 in combination with plasma. The ionizing radiation energy from plasma excites the water molecules and causes these to dissociate into excitation species such as atomic and molecular radicals18. These reactive radicals set up disruptive ionic flux within the biofilms that alters the DNA molecules of spores and other bioentities to effectively annihilate them within and outside the biofilm islands. Around the plasma, free electrons gain energy from the imposed electric field and lose this energy through collisions with neutral gas molecules1920. The energy transfer process dissociates cell-contained moisture and leads to the formation of a variety of free radicals and ions that disrupt the DNA. UV photons from plasma increase mutation rate by damaging chromosomes. In the present study, we report the results after using H2O2 with plasma irradiation to achieve quick decontamination in the enclosed chamber. The effect of this combination was studied on the viability of bacterial spores, viruses and breakage of DNA molecules.
Material & Methods
Plasma torch: The plasma generator in use comprised a height-adjustable anode, fabricated from either thoriated tungsten or tantalum carbide 3 mm Φ rod, concentrically proximate to a 25 mm Φ silver cathode, and had primary and secondary annular pathways for gases such as argon, helium, oxygen and nitrogen. During the present work, argon was used. An insulator separated the electrodes20. The plasma discharge of about 250-350 Watts was sustained at relatively lower magnetic co-intensity, in the range of 0.1-1.0 W/cm2. The electron temperature was of 3 eV order of magnitude, and the driving frequency was about 2.45 MHz. This frequency was found effective for dissociating the molecular cell-containing moisture into atomic hydrogen and oxygen. A uniform density of about 1013 electrons per cubic centimetre within a zone of approximately 20 cubic centimetres was generated.
Treatment with H2O2: H2O2 vapour (5% v/v) was introduced to the isolator using vapour generator at atmospheric pressure, with a continuous dynamic flow rate in the range of 30-300 mg/min. Both H2O2 and plasma discharge were initiated within the isolator in an electrically symmetrical configuration at ground potential.
Bacterial spores used: Bacillus subtilis ATCC #9372 spores strips (HiMedia labs, Mumbai) were used in the study. The growth conditions were temperature of 30°C at 24 h in nutrient agar or broth.
Virus strains used: Chikungunya (CHIK) Kolkata strain 634029; Alphavirus and Kyasanur Forest Disease (KFD) virus W1930 strains; flavivirus were used throughout the study. The work was performed in the High Containment Laboratory, National Institute of Virology, Pune, India. The virus stock was prepared in infant Swiss albino mice by intracerebral inoculation. Mice brains inoculated with CHIK and KFD virus were harvested on the 3rd post-infection day. Ten per cent mice brain suspension in 1.25 per cent BAPS (Bovine albumin in Phosphate saline) was prepared from pooled mice brain. The suspension was sonicated and centrifuged at 10,000×g for 10 min. Supernatant was used as stocks after determining the titre (2.7 and 3.5 log MID50/0.02 ml, respectively).
Experiments: Three sets of experiments were performed to understand the effect of only plasma radiation, direct effect of H2O2 and plasma radiation and independent effect of H2O2 and plasma on the bacterial spores, viruses and amplified nucleic acid molecules [polymerase chain reaction (PCR) product].
Effect of plasma radiation on the bacterial spores, viruses and amplified nucleic acid molecules (PCR product): Sets of closed 1.5 ml Eppendorf tubes containing 200 µl of CHIK and KFD virus (titre 3.11 × 106 and 2.67 × 107 pfu/ml, respectively), bacterial spore strips and amplified nucleic acids (815 bp segment of KFD) were kept parallel to the plasma torch at a distance ranging from 1, 3 and 6 cm. Experiments were repeated at intervals of 1, 3, 6 and 10 min. To determine the effect of lethality of plasma, after exposure, the viruses were inoculated in Vero cells after log dilutions and observed for seven days. The spore strips were incubated in the medium recommended by the manufacturers and observed for 3-4 days. Immediately afterwards, the nucleic acid molecules were run on the agarose gel to determine any breakage in the DNA fragments. Part of the exposed product was also re-amplified by PCR using log dilution21. For the controls, unexposed PCR products were also processed similarly.
Direct effect of H2O2 and plasma on the bacterial spores, viruses and DNA: This experiment was similar to the above-mentioned experiment. The viral pathogens and PCR product were kept in either open 1.5 ml Eppendorf tubes or 96-well flat bottom microtitre plates while B. subtilis strips were kept on Petri plates. The exposed material was also analyzed similarly as mentioned above.
Independent effect of H2O2 and plasma on the bacterial spores, viruses and DNA: The experiment as mentioned above was individually repeated where either of the system was used in the isolators to assess the effect.
Results
Effect of plasma radiation on the bacterial spores, viruses and amplified nucleic acid molecules (PCR product): Experiments performed to determine the lethal effect of plasma radiation on the B. subtilis spores did not show loss of viability. Similarly, there was no effect on both viruses as it showed cytopathic effect (CPE) in Vero cells on the 3rd post-infection day onwards. The PCR on the nucleic acid product of KFD virus which was exposed to plasma radiation showed amplification of expected size of PCR products while aliquot of exposed nucleic acid PCR product run on agarose gels did not show breakage in the DNA.
Direct effect of H2O2 and plasma on the bacterial spores, viruses and DNA: Experiments performed to determine the direct lethal effect of plasma radiation in combination with the effect of H2O2 on the bacterial spores showed total loss of viability of spores on the strips after three minutes. The exposed suspension containing the viruses in the Eppendorf tubes and 96-well plates did not show reduction of CPE, suggesting that these were in suspension, forming about 10 mm layer, and this exposure did not have complete penetration. The PCR products, which were exposed, showed amplification of expected size of product, and agarose gels with and without exposure of PCR products did not show breakage in the DNA (Table).
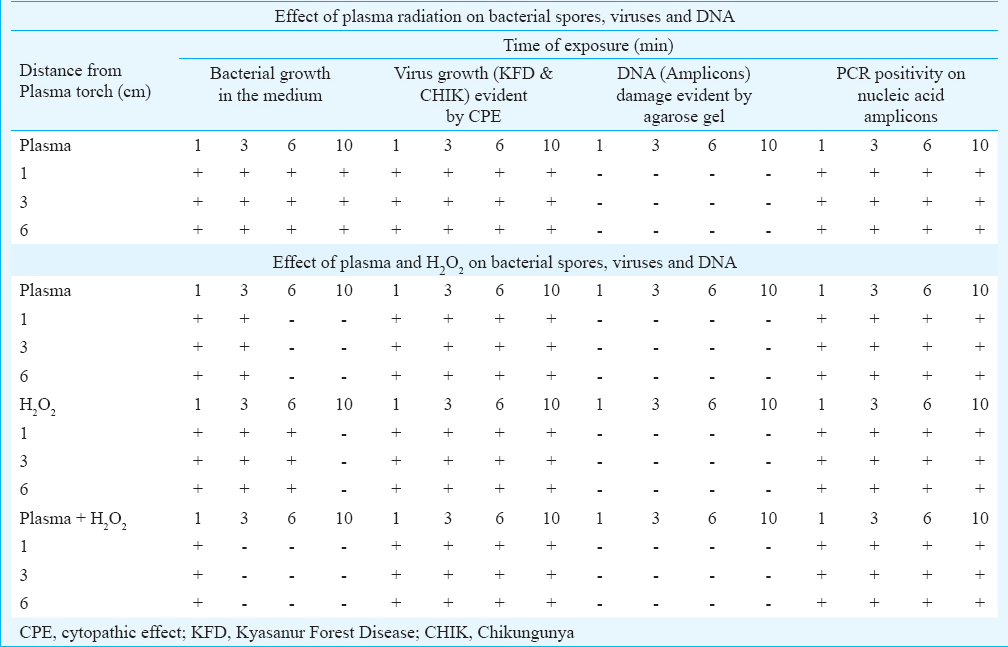
Independent effect of H2O2 and plasma on the bacterial spores, viruses and DNA: Experiments performed to determine the lethal effect independently for both H2O2 and plasma on the bacterial spores showed that plasma alone took six minutes while H2O2 alone took 10 min to show total loss of viability of spores on the strips. However, up to three minutes, exposure of both plasma and H2O2 did not show reduction of CPE and had low penetration since these were in solution, forming about 10 mm layer. Similar results were recorded with PCR products, and after six minutes of the exposure, the amplicons could be amplified (Table).
Discussion
Plasma radiation has not been used for decontamination in the laboratories working on biohazardous material. However, use of H2O2 in such laboratories has been advocated, which also has its own advantages and disadvantages22. Our results showed that when both systems, i.e. plasma and H2O2, were used together for three minutes, these acted as good biocides for sterilization of both surface and contaminated air present in the closed chamber. Data also suggested that although it showed damage of spores present on the strips, it did not show any penetration power since the virus present in the vials and microtitre plates could not be destroyed. The advantage of this method is that after achievement of sterilization of enclosed chambers, they become available for use after a few minutes23. Another advantage of this method is that there is no need of even wiping any of the outer surfaces, bottles caps, etc. with spirit/bleach/lysol or any other disinfectant before use. It is also useful in tissue culture where closed bottles of media, etc. are kept and there are no damaging effects of radiation in such a short period. In addition, fumigation of the chamber can be achieved in a few minutes.
The disadvantages of this method are that the inlet and outlet ducts need modification in the isolators and biosafety cabinets for re-circulation of H2O2 and plasma for a few minutes. The use of plasma for longer durations can cause low level of melting of inner surfaces of gloves. Therefore, plasma irradiation needs further detailed studies from biosafety point of view before it can be used in practice. In conclusion, our findings show that this method of using a combination of H2O2 vapour and plasma irradiation is promising for achieving surface sterilization in a reasonably short time. This application may be useful in pharmaceutical industry, particularly which are required to work on clean facility concept.
Acknowledgment
The authors thank Dr V. M. Katoch, Former Secretory, Department of Health Research, and Director General, Indian Council of Medical Research, New Delhi, for his support.
Conflicts of Interest: None.
References
- Keith FA, ed. CRC handbook of laboratory safety (5th ed). New York: CRC Press; 2000.
- Gaseous decontamination methods in high-containment laboratories. Appl Biosafety. 2012;17:1.
- [Google Scholar]
- 2004. Public Health Agency of Canada. Decontamination. The laboratory biosafety guidelines. (3rd ed). Available from: http://www.phac-aspc.gc.ca/publicat/lbg-ldmbl-04/ch8-eng.php
- Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J Hosp Infect. 2006;62:149-55.
- [Google Scholar]
- Vapor-phase hydrogen peroxide as a surface decontaminant and sterilant. Appl Environ Microbiol. 1990;56:503-6.
- [Google Scholar]
- Sterilization. In: Ayliffe GAJ, Fraise AP, Geddes AM, Mitchell K, eds. Control of hospital infection: A practical handbook (4th ed). New York: Oxford University Press; 2000. p. :381.
- [Google Scholar]
- United States Patent Preparation of Bis-Chloromethyl Ether Saul R. Buc, Easton, Pa., assignor to General Aniline and Film Corporation, New York, A Corporation of Delaware No Drawing. Application, Serial No. 282,457; 15 April 1952
- Biosafety in microbiological and biomedical laboratories. 1999. U. S. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Institutes of Health. (4th ed). Washington, DC: U.S. Government Printing Office; Available from: http://www.cdc.gov/od/ohs/biosfty/bmbl4/bmbl4toc.htm
- [Google Scholar]
- Primary containment for biohazards: selection, installation and use of biological safety cabinets. 2000. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, and National Institutes of Health. (2nd ed). Washington, DC: U.S. Government Printing Office; U.S. Available from: http://www.cdc.gov/od/ohs/biosfty/bsc/bsc.htm
- [Google Scholar]
- Characterization of UV-peroxide killing of bacterial spores. J Food Prot. 2003;66:1233-40.
- [Google Scholar]
- Comparison of the disinfection effects of vacuum-UV (VUV) and UV light on Bacillus subtilis spores in aqueous suspensions at 172, 222 and 254 nm. Photochem Photobiol. 2010;86:176-81.
- [Google Scholar]
- Bacterial endospore inactivation caused by outgassing of vapourous hydrogen peroxide from polymethyl methacrylate (Plexiglas®) Lett Appl Microbiol. 2007;45:485-90.
- [Google Scholar]
- Prion inactivation using a new gaseous hydrogen peroxide sterilisation process. J Hosp Infect. 2007;67:278-86.
- [Google Scholar]
- Efficacy of dry mist of hydrogen peroxide (DMHP) against Mycobacterium tuberculosis and use of DMHP for routine decontamination of biosafety level 3 laboratories. J Clin Microbiol. 2008;46:2955-8.
- [Google Scholar]
- Assessing the biological efficacy and rate of recontamination following hydrogen peroxide vapour decontamination. J Hosp Infect. 2007;67:182-8.
- [Google Scholar]
- Decontamination assessment of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surfaces using a hydrogen peroxide gas generator. J Appl Microbiol. 2005;99:739-48.
- [Google Scholar]
- Use of hydrogen peroxide vapor for deactivation of Mycobacterium tuberculosis in a biological safety cabinet and a room. J Clin Microbiol. 2007;45:810-5.
- [Google Scholar]
- Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm. 2001;226:1-21.
- [Google Scholar]
- Nonthermal decontamination of biological media by atmospheric-pressure plasmas: review, analysis, and prospects. IEEE Trans Plasma Sci. 2003;30:1409-15.
- [Google Scholar]
- Comparative study on the use of different metal electrodes in low-pressure glow discharge plasma sterilization. Plasma Med. 2014;4:1-10.
- [Google Scholar]
- Kyasanur forest disease diagnosis by nested reverse transcription polymerase chain reaction, TaqMan based qRT PCR and IgM capture ELISA. J Virol Methods. 2012;186:49-54.
- [Google Scholar]
- Cold atmospheric air plasma sterilization against spores and other microorganisms of clinical interest. Appl Environ Microbiol. 2012;78:5077-82.
- [Google Scholar]
- Room temperature sterilization of surfaces and fabrics with a one atmosphere uniform glow discharge plasma. J Ind Microbiol Biotechnol. 1998;20:69-74.
- [Google Scholar]






