Translate this page into:
Fatal infection in adults by pneumolysin & autolysin producing, non-vaccine serotype Streptococcus pneumonia
‡ For correspondence: reba.kanungo@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Despite variable levels of antibiotic resistance in clinical isolates of Streptococcus pneumoniae reported from India, invasive pneumococcal infection continues to pose a challenge in children1. It is a paradox that the susceptibility of pneumococci to antibiotics is not as major a problem as it is observed with staphylococci, enterococci and the Gram-negative bacteria, though outcome of some of the pneumococcal infections are fatal2. Rapidly progressive disease in the vulnerable population namely, the very young and the elderly is a challenge to the treating physician, a factor which is compounded by lack of confirmatory diagnosis due to poor laboratory support. Sporadic reports of adult pneumococcal infections have been documented3. Factors predisposing this group to fatal pneumococcal infection are unknown4. Vaccination of the adult population is limited to the very elderly and those with risk of underlying immunosuppression and splenectomized persons5. We encountered two cases of invasive pneumococcal infections in adult patients with progressive illness with a fatal outcome in a tertiary care centre in south India during 2012. An attempt was made to detect virulence genes coding for pneumolysin (ply) and autolysin (lytA), as these two antigens are known to be associated with virulence of S. pneumoniae in experimental animals6.
The first case was a 72 years old male with loss of consciousness for 12 h, brought to the emergency unit of Pondicherry Institute of Medical Sciences, Puducherry, India. He had no history of fever, breathlessness or seizures. He did not have any known risk factors. At admission he had respiratory distress and was transferred to the intensive care unit (ICU) for intubation. Chest X- ray showed bilateral infiltrates with pneumothorax on the left side. Computed tomography (CT) of the brain revealed dilated ventricles. Initial laboratory investigations revealed the following values: total WBC count of 9800/mm3, differential count of 89 per cent neutrophils, 8 per cent lymphocytes and 3 per cent monocytes. A preliminary diagnosis of sepsis with pneumonia and pyogenic meningitis was made. He was started empirically on cefoperazone sulbactam, metronidazole and doxycycline (due to the area being endemic for scrub typhus). Subsequently, blood and CSF cultures grew S. pneumoniae (IBT 1960) (serotyped as 33C- Pneumotest- Statens Serum Institute, Solna, Sweden), which was susceptible to ampicillin, penicillin (by oxacillin screen), ceftriaxone, cefotaxime and vancomycin. Following the culture report antibiotics were changed to vancomycin and ampicillin. He developed hypotension and shock, and despite ventilatory support, administration of intravenous fluids, antibiotics and vassopressor agents his condition deteriorated and he died within 48 h of admission.
The second case was a 47 year old male who was conscious but restless and disoriented, and was brought to the casualty of this hospital. There was history of fever and shortness of breath for two weeks, cough for one week and chest pain for two days. There was no other co-morbid condition. Chest X-ray revealed right lower lobe consolidation. Complete blood count revealed total WBC count to be 24000/mm3 and a differential count suggestive of pyogenic infection (95% neutrophils, 4% lymphocytes, 1% monocytes). A diagnosis of right lower lobe pneumonia associated with acute respiratory distress syndrome, sepsis and multi-organ dysfunctional syndrome was made. He was empirically started on linezolid, imipenem and piperacillin-tazobactam. Sepsis was confirmed by a positive blood culture for S. pneumoniae (IBT -1975; serotype 7C), which was sensitive to penicillin, ciprofloxacin, ceftriaxone, erythromycin, gentamicin and vancomycin. The patient was continued with linezolid, imipenem while azithromycin was added. His condition continued to deteriorate and he succumbed to the infection within 72 h of admission.
The two isolates were further tested for the presence of different virulence determinants such as genes encoding for pneumolysin (ply), autolysin (lytA) and to document presence of virulence factors and penicillin binding protein (pbpA)7. The primer details are given below: lytA (size 319 bp)4 forward primer F-5’AACCGTACAGAATGAAGCGG-3’ and reverse primer R-5’ TATTCGTGCAATACTCGTGCG-3’; ply (size 348 bp) forward primer F- 5’ATTTCTGTAACAGCTACCAACGA3’ and reverse primer R- 5’GAATTCCCTGTCTTTTCAAAGTC3’; pbpA (size-789 bp) forward primer F-5’CCGTATCCTGGGAGCTTTCTT-3’ and reverse primer R-5’-TCGCGGTTTGTTTCTACTGC-3’. All primers used in the study were designed using GeneTool software and custom synthesize by Eurofins (Bengaluru, India), used in previously described study7. All three genes were detected in both the isolates (Figure).
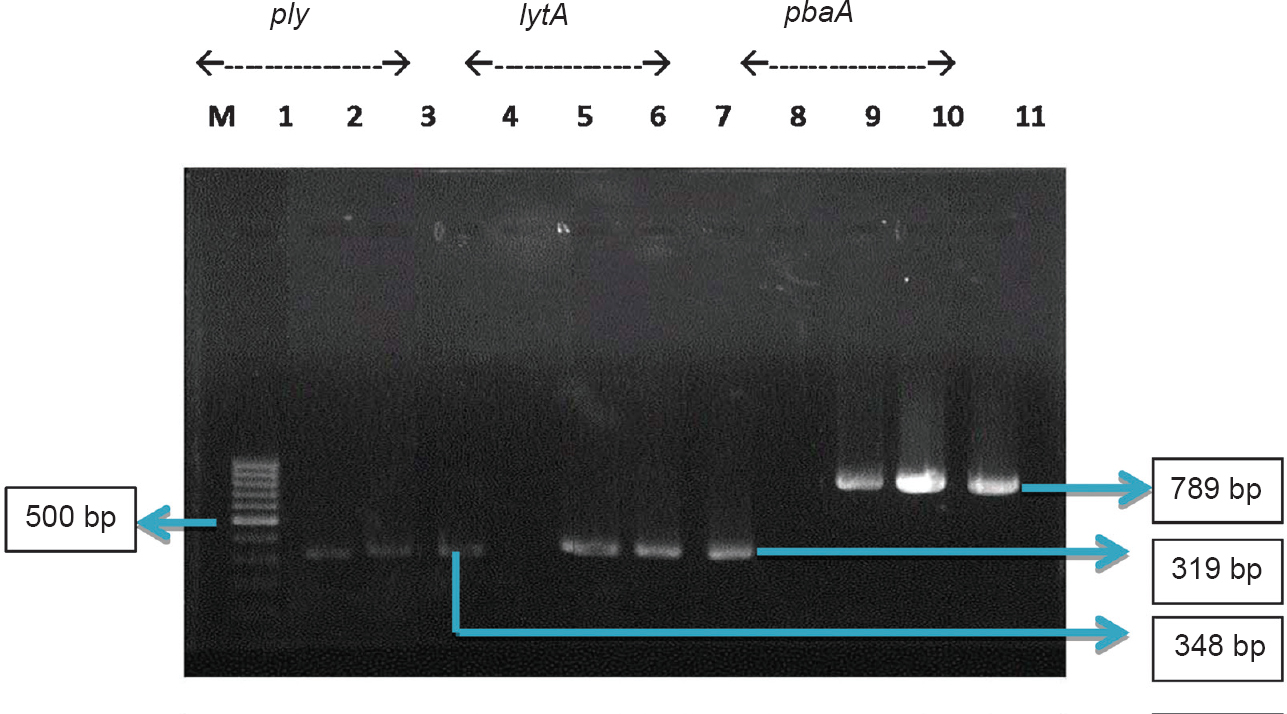
- Pneumolysin (ply), autolysin (lytA) and modified penicillin binding protein (pbpA) genes amplified in the two isolates from cases of fatal pneumococcal infection. Amplicon sizes: ply - 348 bp; lytA - 319 bp and pbpA - 789 bp. M - 100 bp ladder (Gene ruler); Lanes 1, 5, 9; IBT-1960 (serotype 33C); Lanes 2, 6,10 - IBT -1975 (serotype 7C) and Lanes 3,7,11 - S. pneumoniae ATCC 49619; Lanes 4, 8, negative control.
Rapidly fatal pneumococcal disease is known to occur in immunocompromised individuals, splenectomized individuals and HIV infected patients are at increased risk of developing severe pneumococcal disease8,9. Fatal outcome in the immunocompetent patients have been infrequently reported in literature10. Rapidly progressing pneumococcal sepsis with metabolic acidosis and disseminated intravascular coagulation has been reported in two adult individuals by Iinuma et al3.
Analysis of the isolates revealed two interesting observations. Both the isolates that were responsible for fatal outcome belonged to non-vaccine serotypes i.e. 33C and 7C. The capsular polysaccharide-23 valent vaccine used for adult vaccination does not contain these two serotypes1112. Studies in the West have shown that infections due to vaccine serotypes have declined in adults following vaccination of children with PCV-7 while non-vaccine serotypes causing invasive infections in high risk adults has risen131415. In India, where pneumococcal vaccines have not been included in the routine immunization schedule in children, and adult vaccination is sporadic, and serotyping of invasive isolates remains a challenge. Routine typing to know the incidence of infections due to vaccine or non-vaccine serotypes both in children and adults, is not undertakn by most clinical laboratories across the country. Fatal infections caused by non-23 valent vaccine serotypes such as 33C and 7C of S. pneumoniae observed in this study raise the question on current strategies of adult pneumococcal vaccination. As the patients were healthy adults, preventive vaccination would not have played a role in protecting these individuals.
Pathogenesis of invasive pneumococcal disease depends on both host inflammatory response as well as the organism's virulence. Several virulence factors have been incriminated in the pathogenesis of pneumococcal disease, including autolysin, pneumolysin (a key inducer of apoptosis) and pneumococcal surface adhesin A (PsaA)16. Capsular polysaccharide is the most commonly attributed factor. Pneumolysin is a cholesterol dependent cytolysin, that binds to cells and induces apoptosis17. Pneumolysin is known to reduce ciliary action, phagocytic function of polymorphonuclear cells and induce acute inflammatory reaction18. Both the isolates in this study were positive for genes coding for autolysin and pneumolysin. Though pbp gene was detected, penicillin resistance was not detected phenotypically, suggesting non-phenptypic expression of the gene.
Our observation highlights two points. First, the involvement of non-vaccine serotypes in fatal invasive pneumococcal infection in adults. Some studies have shown a decline in adult pneumococcal disease following introduction of multi-valent pneumococcal conjugate vaccine in children13,18. But adult invasive infection and protecting the high risk groups still remains a major problem in several countries19. The US advisory committee on invasive pneumococcal disease (ACIP) has recommended the use of 23 valent polysaccharide vaccine (PPSV23) and the PCV13 (conjugate vaccine) for use in the elderly population aged >65 yr7,20. Secondly, could presence of pneumolysin and autolysin genes in the isolates obtained from these patients have any diagnostic or prognostic indications? Severity of pneumococcal pneumonia associated with bacterial genomic load determined by the copies of lytA genes has been reported21.
In patients with no underlying risk factors, a strong suspicion of invasive pneumococcal disease with rapid assessment needs to be done to prevent grave outcome. A delay in treatment can occur in young and middle-aged patients, who are otherwise healthy leading to serious consequence. Accurate management in a timely manner can improve outcome. Rapid laboratory tests are essential for confirmatory diagnosis. A rapid diagnostic tool has been developed to detect pneumococcal antigen in urine and other body fluids which shows high sensitivity and specificity in adults19. The two isolates from our patients had the lytA and ply genes, although we did not attempt to detect this from the patients’ blood or body fluids. High level of clinical suscpicion with laboratory support by conventional blood culture, effective case management with appropriate antibiotics will decrease morbidity and mortality due to invasive pneumococcal disease in adults.
Acknowledgment
The authors thank Dr K.L. Ravikumar, Principal Investigator- PIDOPS STUDY KIMS Hospital and Research Centre, Bengaluru for serotyping the isolates.
Conflicts of Interest: None.
References
- Circulating serotypes and trends in antibiotic resistance of invasive Streptococcus pneumoniae from children under five in Bangalore. J Clin Diagn Res. 2013;7:2716-20.
- [Google Scholar]
- Invasive pneumococcal disease associated with high case fatality in India. J Clin Epidemiol. 2013;66:36-43.
- [Google Scholar]
- Rapidly progressive fatal pneumococcal sepsis in adults: a report of two cases. J Infect Chemother. 2007;13:346-9.
- [Google Scholar]
- Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:145-54.
- [Google Scholar]
- Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46:1.
- [Google Scholar]
- Loop-mediated isothermal amplification method targeting the lytA gene for detection of Streptococcus pneumoniae. J Clin Microbiol. 2005;43:1581-6.
- [Google Scholar]
- Detection of pneumolysin and autolysin genes among antibiotic resistant Streptococcus pneumoniae in invasive infections. Indian J Med Microbiol. 2010;28:34-9.
- [Google Scholar]
- Pneumococcal disease prevention among adults: Strategies for the use of pneumococcal vaccines. Am J Prev Med. 2015;49(Suppl 4):D60-5.
- [Google Scholar]
- Pneumococcal colonisation density: a new marker for disease severity in HIV-infected adults with pneumonia. BMJ Open. 2014;4:e005953.
- [Google Scholar]
- Primary Streptococcus pneumoniae pericarditis. Proc (Bayl Univ Med Cent). 2013;26:35-8.
- [Google Scholar]
- Pneumococcal polysaccharide vaccine efficacy: an evaluation of current recommendations. JAMA. 1993;270:1826-31.
- [Google Scholar]
- Serotype distribution of Streptococcus pneumoniae infections among preschool children in the United States, 1978-1994: Implications for development of a conjugate vaccine. J Infect Dis. 1995;171:885-9.
- [Google Scholar]
- Epidemiology of invasive pneumococcal disease among high-risk adults since the introduction of pneumococcal conjugate vaccine for children. Clin Infect Dis. 2013;56:59-67.
- [Google Scholar]
- Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32-41.
- [Google Scholar]
- Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784-92.
- [Google Scholar]
- Streptococcus pneumoniae evades human dendritic cell surveillance by pneumolysin expression. EMBO Mol Med. 2009;1:211-22.
- [Google Scholar]
- Current concepts in host-microbe interaction leading to pneumococcal pneumonia. Curr Opin Infect Dis. 2013;26:277-83.
- [Google Scholar]
- Protection against bacteremic pneumococcal infection by antibody to pneumolysin. J Infect Dis. 2001;183:827-30.
- [Google Scholar]
- Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA. 2005;294:2043-51.
- [Google Scholar]
- The remaining challenges of pneumococcal disease in adults. Eur Res Rev. 2012;21:57-65.
- [Google Scholar]
- Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest. 2009;136:832-40.
- [Google Scholar]





