Translate this page into:
Compromised zinc status of experimental rats as a consequence of prolonged iron & calcium supplementation
Reprint requests: Dr Kalpana Platel, Department of Biochemistry & Nutrition, CSIR-Central Food Technological Research Institute, Mysore 570 020, Karnataka, India e-mail: kalpanaplatel@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Iron supplementation is usually given to pregnant and lactating women who may also have marginal deficiency of zinc. The negative impact of supplemental iron and calcium on zinc status is a cause of concern. The present investigation was undertaken to examine the effect of inclusion of iron and calcium in the diet at supplementary levels on zinc status of experimental rats.
Methods:
Groups of experimental rats were maintained on diets supplemented with iron (Molar ratio - Zn:Fe 1:30) and calcium (Molar ratio - Zn:Ca 1:667) both individually and in combination for six weeks. Zinc status of these rats was assessed by determining zinc concentration in circulation and in organs, and the activities of zinc containing enzymes in serum and liver.
Results:
The zinc status of experimental rats receiving supplemental levels of iron and calcium was significantly compromised. Zinc concentration in serum, kidney, spleen and liver was reduced significantly by both these minerals. Six weeks of supplementation of iron and calcium individually, significantly reduced the activity of liver and serum superoxide dismutase and alkaline phosphatase. Activity of liver alcohol dehydrogenase was lowered in calcium supplemented group and in calcium + iron supplemented group, while that of carbonic anhydrase was significantly reduced by iron, calcium and their combination.
Interpretation & conclusions:
Supplemental levels of iron and calcium, both individually and in combination, significantly compromised the zinc status of experimental rats. This negative effect of these two minerals was more prominent when these were supplemented for a period of six weeks.
Keywords
Calcium
iron
supplementation
zinc-containing enzymes
zinc status
Zinc is an essential element for human health, having diverse physiological functions. The primary dietary sources of zinc are animal foods and, to a lesser extent, food grains. Vegetarian diets contain significant amounts of fiber, phytate, and tannin, compounds that have been found to bind to minerals and reduce their bioavailability1. Apart from these inherent inhibitors of mineral absorption, minerals that are similar in chemical configuration are likely to compete with each other at the site of zinc absorption, thus reducing its bioavailability.
Iron at the molar ratios inherent in food grains probably does not affect the bioavailability of zinc. However, pharmacological supplements of the same may have a bearing on the bioavailability of zinc. Iron supplementation has been reported to inhibit zinc absorption in vitro as well as in human subjects. Similarly, high levels of calcium in the diet have been found to have a negative impact on zinc absorption1234. These negative effects have been seen when the added iron and calcium are far in excess of the inherent zinc concentration, with respect to molar ratios of the minerals.
Consumption of aqueous solution of iron is reported to decrease zinc absorption in humans in a dose-dependent manner not in a meal56. Iron supplements have been reported to decrease zinc absorption in pregnant women7, and in teenage pregnant women taking daily multi-vitamin supplements containing 18 mg iron8. We have earlier reported2 that presence of supplemental levels of iron and calcium brought about a significant reduction in zinc bioaccessibility from a combination of rice (Oryza sativa) and red gram (Cajanus cajan). The negative influence of high exogenous levels of iron and calcium on zinc bioaccessibility was seen in both raw and cooked grains2.
Iron supplementation is usually given to pregnant and lactating women to prevent iron deficiency; however, these women are also likely to have marginal deficiency of zinc. Similarly, it is not uncommon for adult women to take calcium supplements to maintain their bone health. In such cases, the negative impact of supplemental iron and calcium on zinc status would be a matter of concern. The present investigation was undertaken to examine the effect of inclusion of iron and calcium in the diet at supplementary levels on zinc status of experimental rats.
Material & Methods
All fine chemicals and other chemicals used in this study were purchased from Sigma Chemical Co., USA. Ferrous sulphate and calcium carbonate used as sources of iron and calcium, respectively, were purchased from SISCO Research laboratories Pvt. Ltd (Mumbai, India). Casein was purchased from Nimesh Corporation (Mumbai, India).
Animals & experimental diets: The levels of exogenous iron and calcium to be added to the diets were determined on the basis of our earlier finding that iron and calcium at molar ratios of Zn:Fe - 1:30, and Zn:Ca - 1:667, significantly inhibited the bioaccessibility of zinc from a cereal-pulse combination2. These levels of iron and calcium correspond to 60 mg of iron and 1000 mg of calcium per day that are generally prescribed as supplementary doses. Ferrous sulphate and calcium carbonate were used in the study as the sources of iron and calcium, respectively. The salts were added in such quantities as to maintain a molar ratio of inherent zinc content of the diet to the added iron and calcium at Zn:Fe = 1:30, and Zn:Ca = 1:667.
The composition of the grain-based basal control diet was: wheat flour - 26.7 per cent, finger millet flour - 26.7 per cent, chickpea flour - 26.7 per cent, casein - 6.67 per cent, refined groundnut oil – 10.0 per cent, shark liver oil - 0.67 per cent, common salt - 2 per cent. This control diet was adequate in terms of zinc content. The test diets were prepared by adding iron and calcium salts to the basal control diet in the following variations: (i) basal control diet + ferrous sulphate; (ii) basal control diet + calcium carbonate; and (iii) basal control diet + ferrous sulphate + calcium carbonate.
Adult female Wister rats (8 wk old; weighing 100-110g) [OUT-Wister, IND-cft (2c)] obtained from Experimental Animal Production Facility of CSIR-Central Food Technological Research Institute, Mysore, India, were used in this study. The animal experiments were carried out at the same facility, with due approval from the Institutional Animal Ethics Committee.
The animals were placed in individual cages in an approved animal house facility with 12: 12 h light and dark cycle with temperature 25 ± 2°C. Groups of rats (n = 6 rats per group) were maintained on the control and experimental diets for a period of four to six weeks. Body weight of the animals was monitored at weekly intervals. One batch of animals was sacrificed at the end of four weeks and one batch at the end of six weeks of supplementation. At the end of the two time intervals, the rats were sacrificed under light ether anaesthesia. Blood was collected by cardiac puncture. Serum was separated by centrifugation and stored frozen. Organs such as liver, spleen and kidney were quickly excised, washed in ice-cold normal saline, blotted dry and weighed. The organs were stored frozen pending analysis. Femur and tibia were cleaned to remove adhering tissues and weighed.
Activity of zinc containing enzymes: The liver samples were homogenized with 0.9 per cent saline to prepare a 10 per cent homogenate, which was used with appropriate dilutions for the enzyme assays. Superoxide dismutase (SOD) activity in serum and liver was measured by quantifying the inhibition of cytochrome-C reduction in xanthine-xanthine oxidase system as described by Flohe & Otting9; the reaction was initiated by the addition of xanthine oxidase to the reaction mixture containing cytochrome-C. Alcohol dehydrogenase activity was determined in liver by the method of Brink et al10, with slight modification, based on spectrophotometric measurement (340 nm) of the amount of nicotinamide adenine dinucleotide being reduced at pH 9.6 in the presence of excess ethanol. Carbonic anhydrase in liver was assayed by a spectrophotometric method involving the measurement of the amount of p-nitro phenyl acetate being hydrolyzed to p-nitro phenol at pH 7.611. Alkaline phosphatase activity in serum and liver was assayed by estimating the amount of p-nitro phenol liberated in unit time as determined in alkaline pH at 405 nm12. Protein in liver was estimated by the method of Lowry et al13.
Determination of zinc content in tissues and bones: Zinc content in serum was determined by Atomic Absorption Spectrometry after appropriate dilution with 8 per cent butanol in deionized water. Tissues and bone samples were ashed in a muffle furnace at 550 °C for 24 h and the ash was dissolved in concentrated hydrochloric acid. Zinc content was determined by Atomic Absorption Spectrometry (Shimadzu AAF-6701, Japan). Calibration of the zinc measurement was performed using zinc standards and appropriate acid blanks. The reproducibility values were within 2.0 per cent. All measurements were carried out with standard flame operating conditions as recommended by the manufacturer.
Statistical analysis: The comparisons between the groups was made by unpaired Student's t test. Six rats were included in each group, and the results are expressed as mean ± SEM of six values.
Results
Table I presents the gain in body weight, bone weights and organ weights of the experimental animals at the end of the feeding regimen. Four weeks supplementation with iron, calcium and their combination brought about a decrease in the gain in weight of the experimental rats. The reduction in gain in body weight was 17, 23 and 47 per cent in the rats supplemented with iron, calcium and their combination, respectively. Similar reduction in gain in body weight was seen in rats supplemented with calcium (22.5% reduction) and the combination of iron and calcium (25.7% reduction) for six weeks.
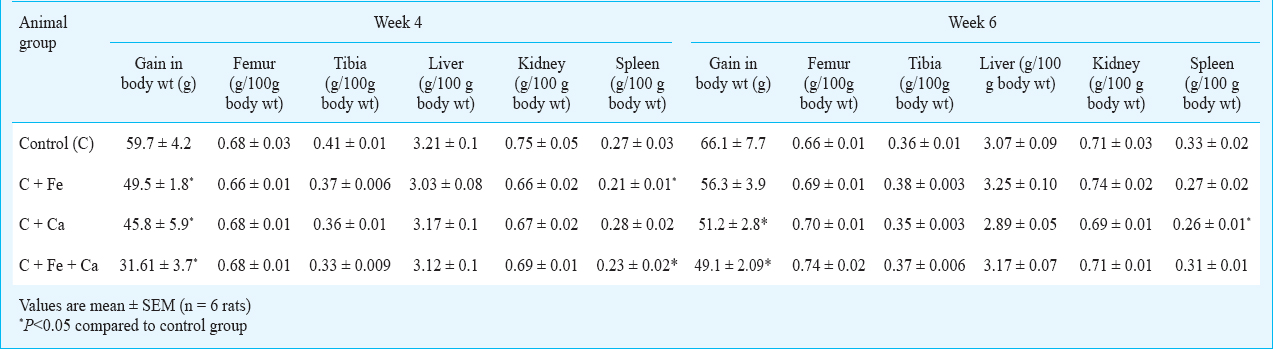
Supplementation with iron, calcium and their combination for four weeks did not have any significant effect on the weight of femur, while the weight of tibia was reduced by 20 per cent by the combination of iron and calcium, and marginally reduced by iron (9.7%) and calcium (12%). Similar to the four-week supplementation, these minerals did not bring about any significant change in the bone weight upon supplementation for six weeks, although a slight, insignificant increase in the weight of femur was seen upon supplementation with calcium and combination of calcium and iron. However, these reductions in either body weight gain or bone weight may not be of much consequence in these adult rats, which are past the period of rapid growth. Supplementation with iron and the combination of iron and calcium for four weeks brought about a significant reduction in the weight of spleen (22% reduction), while a marginal reduction in the weight of kidney (around 12%) was seen in animals supplemented with iron and calcium independently (Table 1). Supplementation with these minerals for six weeks did not have any effect on the weight of either liver or kidney, while the weight of spleen was reduced to an extent of 18 per cent by iron and 21 per cent by calcium.
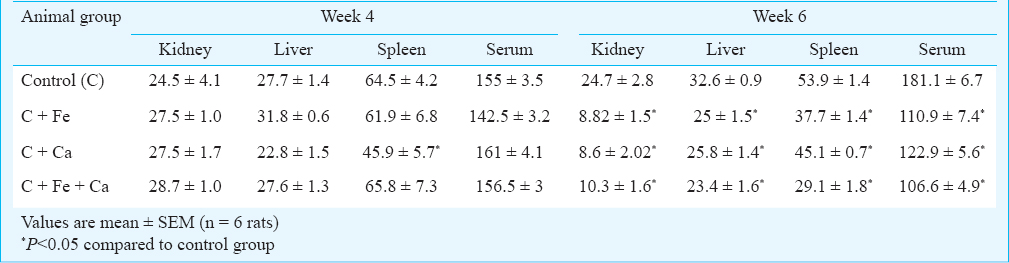
Table II presents zinc concentration in the organs and serum of animals maintained on supplemental iron, calcium and iron+calcium for four and six weeks. Supplementation with calcium for four weeks reduced the zinc concentration in spleen to an extent of 29 per cent, while the zinc concentration of the other organs was not affected by either iron, calcium or their combination. On the other hand, there seems to be a slight, insignificant increase in the zinc concentration in kidneys of animals given diets supplemented with these minerals. However, when the supplementation with iron, calcium and their combination was continued up to six weeks, zinc concentration in liver, kidney and spleen was significantly reduced. Zinc concentration in kidney was reduced by 64, 65 and 58 per cent by iron, calcium and their combination, respectively. Liver zinc was reduced to an extent of 23, and 20 per cent by iron and calcium, while their combination lowered the same by 28 per cent. Iron brought about a 30 per cent reduction in spleen zinc concentration, while the reduction in the same by calcium was not high (16%). However, the combination of iron and calcium reduced the concentration of zinc in spleen by 46 per cent. Supplementation with these minerals either individually or in combination for four weeks did not have any negative effect on zinc concentration of serum. However, six week supplementation significantly reduced serum zinc concentration. The extent of reduction was 39, 32 and 41 per cent by supplemental levels of iron, calcium and their combination, respectively (Table II).
Concentration of zinc in femur of the rats maintained on diets supplemented with iron, calcium and their combination is presented in the Figure. Four weeks of supplementation with either of the minerals did not affect bone zinc concentration. At the end of six weeks, supplementation with iron and the combination of iron and calcium reduced femur zinc concentration by 17.6 and 25 per cent, respectively, while calcium alone did not affect bone zinc concentration.
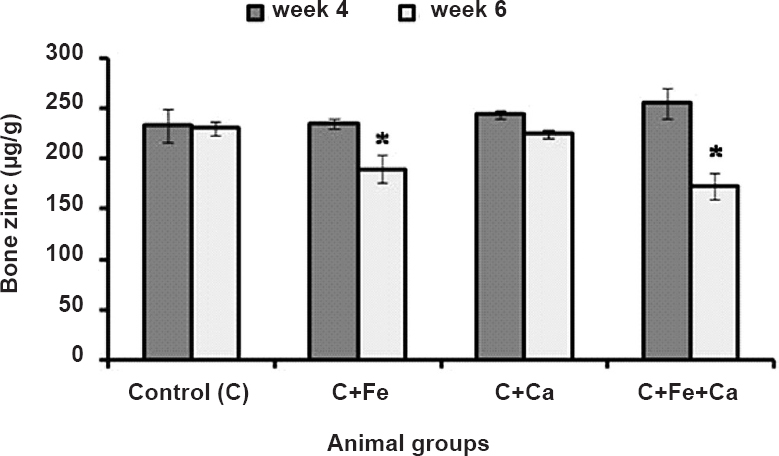
- Zinc concentration in femur bone of rats maintained on supplemental iron, calcium and iron+calcium for four and six weeks. Values are mean ± SEM (n=6 rats). *P<0.05 compared to control group.
The activity of zinc containing enzymes in serum of animals maintained on supplemental iron, calcium and combination of iron and calcium for four weeks was not significantly affected, except for alkaline phosphatase, whose activity was reduced to an extent of 27 per cent by calcium (Table III). There was a slight insignificant increase in the activity of serum SOD by supplementation with these minerals. Exogenous iron and calcium had a negative effect on the activities of zinc-containing enzymes in serum at the end of six weeks of supplementation (Table III). The activity of serum SOD was reduced to an extent of 38 and 25 per cent by calcium and the combination of iron and calcium, respectively, while iron alone reduced the activity of this enzyme by only 10 per cent. The activity of alkaline phosphatase in serum was significantly (P<0.05) decreased by iron and calcium, both individually and in combination. Iron reduced the activity of this enzyme by 50 per cent, while calcium and the combination of iron and calcium decreased the activity by 32 and 47 per cent, respectively.
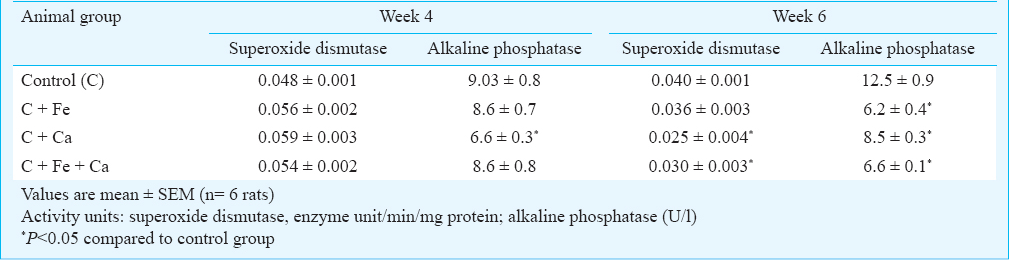
The influence of iron, calcium and their combination on the activity of the zinc-containing enzymes in liver is presented in Table IV. Similar to serum SOD, there was a slight increase in the activity of SOD in liver of the animals supplemented with iron, calcium and their combination for four weeks, the extent of increase being 9.8, 19.6 and 18.9 per cent, respectively. A similar marginal increase in the activity of liver alcohol dehydrogenase was also observed in the animals receiving supplementation with iron and calcium. Supplementation with iron for four weeks brought about a significant decrease in the activity of liver alkaline phosphatase, the per cent decrease being 38; while calcium alone did not negatively affect this enzyme, the combination of iron and calcium reduced its activity by 48 per cent. The combination of iron and calcium, when given for four weeks, also brought about a significant decrease in the activity of liver carbonic anhydrase (35.9% decrease), while iron reduced the activity of this enzyme by 20 per cent (Table IV). Supplementation with iron and calcium for six weeks, however, had a significant lowering effect on the activities of all the zinc-containing enzymes in the liver. The negative influence on the activity of SOD was more prominent by iron, which lowered the enzyme activity by 56 per cent, while calcium brought down the activity by about 45 per cent. The combination of iron and calcium reduced the enzyme activity by 37 per cent. Iron and calcium in combination reduced the activity of alkaline phosphatase by 59 per cent, while iron and calcium individually reduced this enzyme activity by 47 and 35 per cent, respectively. Supplementary level of iron did not have any influence on the activity of alcohol dehydrogenase, while it reduced the activity of carbonic anhydrase by 41 per cent; however, calcium brought down the activity of these two enzymes by 39 and 46.5 per cent, respectively. While the activity of alcohol dehydrogenase was reduced to an extent of 38 per cent by the combination of iron and calcium, the reduction in the activity of carbonic anhydrase was significantly (P<0.05) higher (57%) by this combination.

Discussion
The interactions between micronutrients can mutually interfere with their absorption. Chemically similar minerals compete with each other for uptake mechanisms, thus hindering absorption. The extent to which these interactions will hinder absorption will depend on the relative concentrations of the minerals. Iron to zinc ratios of 2:1 or greater were found to have a negative effect on zinc nutriture in humans14. A significant reduction in zinc absorption was seen in human subjects when iron was added to a zinc solution at the molar ratio of 25:1 (Fe:Zn); however, when the iron was included in a meal at the same molar ratio, such a negative effect on zinc absorption was not evident15. Addition of exogenous calcium in the form of either milk or calcium carbonate (Zn:Ca molar ratio 1:126) to the diet of post-menopausal women for 36 days significantly reduced zinc absorption in these women. The negative effect of excess calcium on zinc absorption was also seen when excess calcium was added to a single test meal, where the Zn: Ca molar ratio was 1:134. Calcium added at this level decreased the absorption of zinc by 50 per cent in human subjects. Addition of zinc to the same meal to give a Zn:Ca molar ratio of 1:65, however, countered the negative effect of calcium16. Thus, the interaction between different minerals affecting their bioavailability seems to depend largely on the molar ratios in which they coexist.
Earlier studies in our laboratory showed that exogenous iron or calcium added at a level up to four times the intrinsic concentration to a cereal-pulse combination did not have any negative effect on zinc bioaccessibility from the food grains. However, when iron and calcium were added at supplementary levels to give molar ratios of Zn:Fe 1:30 and Zn:Ca 1:667 to the same cereal-pulse combination, there was a significant reduction in zinc bioaccessibility2.
In the present investigation, there was a significant reduction in the zinc status of experimental rats fed on diets containing supplemental levels of iron and calcium. This effect was especially prominent at the end of six weeks of supplementation. The reduction in zinc status of these rats was reflected in the decreased levels of zinc in serum, kidney, liver, spleen and femur bone. Although a decrease in gain in body weight was observed when the rats were supplemented with iron, calcium and their combination, this may not be of much consequence since the rats used in this study were adults, in whom growth would have been completed. Similarly, the weight of the bones in these animals was not affected by the supplementation with these two minerals, either at four weeks or at six weeks. Iron and calcium had a negative effect on the concentration of zinc in serum and different organs, both independently and in combination, when given for six weeks. Femur zinc concentration was reduced by iron and the combination of iron and calcium, while calcium alone brought about only a marginal decrease. This significant reduction in tissue zinc concentration is indicative of compromised zinc status in the animals given supplementary levels of these two minerals.
The activities of zinc-containing enzymes such as SOD, alcohol dehyrogenase, carbonic anhydrase and alkaline phosphatase in serum and liver were also significantly reduced by supplementary iron and calcium. These two minerals exhibited the negative effects both individually as well as in combination, although the effect of the combination was not additive. Considering the life span of rats, six weeks of supplementation is a prolonged duration during which the negative influence of supplementary levels of iron and calcium on zinc status can easily be discerned. It would be of further interest to see if supplementary levels of iron and calcium will interfere with the repletion of zinc status in zinc-deficient rats when they are given zinc-enriched diets along with exogenous iron and calcium.
The interaction between iron and zinc has been explained by a possible competitive binding to a common transporter protein, divalent metal transporter 1 (DMT1), which is located in the small intestine. It has been postulated that a decrease in the expression of DMT 1 could be the cause for decreased zinc absorption17. Yamaji et al18 have demonstrated that the common transporter for both iron and zinc may be distinct from DMT1. The competitive binding to this transporter could be the reason for compromised zinc uptake.
In conclusion, iron and calcium, when given at supplementary levels, significantly reduced zinc status, probably by inhibiting the absorption of zinc. This interaction is of concern in the background of giving iron and calcium supplements to pregnant women and children, who may already be at risk for deficient zinc status.
References
- Influence of exogenous iron, calcium, protein and common salt on the bioaccessibility of zinc from cereals and legumes. J Trace Elem Med Biol. 2009;23:75-83.
- [Google Scholar]
- Micronutrient interactions: effects on absorption and bioavailability. Br J Nutr. 2001;85:S181-5.
- [Google Scholar]
- Effects of iron, tin, and copper on zinc absorption in humans. Am J Clin Nutr. 1984;40:536-41.
- [Google Scholar]
- Serum zinc changes due to iron supplementation in teen-age pregnancy. Am J Clin Nutr. 1989;50:848-52.
- [Google Scholar]
- Superoxide dismutase assays. In: Lester Packer, ed. Methods in enzymology. Vol 105. New York: Academic Press Inc; 1984. p. :93-104.
- [Google Scholar]
- Modified method for the enzymatic microdetermination of ethanol. Acta Pharmacol et Toxicol. 1954;10:223-6.
- [Google Scholar]
- Esterase activities of human carbonic anhydrases B and C. J Biol Chem. 1967;242:4221-9.
- [Google Scholar]
- Acid and alkaline phosphatase in serum. In: Bergmeyer HU, ed. Methods of enzymatic analysis. New York: Academic Press; 1974. p. :356-60.
- [Google Scholar]
- Competitive interaction of iron and zinc in the diet: consequences for human nutrition. J Nutr. 1986;116:927-35.
- [Google Scholar]
- High dietary calcium intakes reduce zinc absorption and balance in humans. Am J Clin Nutr. 1997;65:1803-9.
- [Google Scholar]
- Iron supplements inhibit zinc but not copper absorption in vivo in ileostomy patients. Am J Clin Nutr. 2003;78:1018-23.
- [Google Scholar]
- Zinc regulates the function and expression of the iron transporters DMT1 and IREG1 in human intestinal Caco-2 cells. FEBS Lett. 2001;507:137-41.
- [Google Scholar]






