Translate this page into:
Pupal productivity & nutrient reserves of Aedes mosquitoes breeding in sewage drains & other habitats of Kolkata, India: implications for habitat expansion & vector management
Reprint requests: Dr Gautam Aditya, Department of Zoology, University of Calcutta, 35 Ballygunge Circular Road, Kolkata 700 019, West Bengal, India e-mail: gautamaditya2001@gmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The quality of breeding sites is reflected through the pupal productivity and the life history traits of Aedes mosquitoes. Using nutrient reserves and pupal productivity of Aedes as indicators, the larval habitats including sewage drains were characterized to highlight the habitat expansion and vector management.
Methods:
The pupae and adults collected from the containers and sewage drains were characterized in terms of biomass and nutrient reserves and the data were subjected to three way factorial ANOVA. Discriminant function analyses were performed to highlight the differences among the habitats for sustenance of Aedes mosquitoes.
Results:
Survey of larval habitats from the study area revealed significant differences (P<0.05) in the pupal productivity of Aedes among the habitats and months. Despite sewage drains being comparatively less utilized for breeding, the pupae were of higher biomass with corresponding adults having longer wings in contrast to other habitats. The nutrient reserve of the adults emerging from pupae of sewage drains was significantly higher (P<0.05), compared to other habitats, as reflected through the discriminant function analysis.
Interpretation & conclusions:
The present results showed that for both Ae. aegypti and Ae. albopictus, sewage drains were equally congenial habitat as were plastic, porcelain and earthen habitats. Availability of Aedes immature in sewage drains poses increased risk of dengue, and thus vector control programme should consider inclusion of sewage drains as breeding habitat of dengue vector mosquitoes.
Keywords
Aedes mosquito
energy reserves
larval habitats
pupal productivity
sewage drain
The dengue vectors Aedes aegypti and Ae. albopictus exploit a wide range of habitats for breeding. The size and shape of such habitats vary extensively ranging from tree-holes1, discarded tyres and containers23 to water storage tanks4. Pupal productivity varies with the available amount of water and resources for larvae5, which is determined by the size and shape of the Aedes breeding habitats6. It has been suggested that the extent of competition among developing larvae is influenced by the water content7 and resource availability8 in the larval habitats. Thus, the habitat type is an important parameter for successful breeding and subsequent emergence as adults. Life history traits, if adult mosquitoes are dependent on the varying levels of feeding and resource acquisition at the larval stage and thus, water and food resources of larval habitats, carry immense significance in the mosquito life cycle9. Hence, the choice and selectivity of oviposition habitat is a crucial factor for continuity of mosquitoes population. For Aedes mosquitoes, the pattern of oviposition habitat selection seems to be more generalized rather than selective in terms of habitat permanence and food resource availability, thereby promoting the cosmopolitan presence of Aedes mosquitoes worldwide10.
Although entomological monitoring has established Aedes as container breeding mosquitoes31011, but in selected instances cesspits and sewage systems have also been found to be exploited by Aedes12131415. Consistent availability of Aedes mosquitoes in cesspits and septic tanks over a period of time in different geographical locations suggests a possible change in oviposition behaviour and breeding preference of Aedes mosquitoes1415. The availability of Aedes in relatively less known habitats112 poses a risk for continued disease transmission, since most of the dengue vector control strategies are based on the established container larval habitats. Exclusion of sewage and cesspits may allow continued breeding and availability of the vectors.
In this study, an assessment of the pupal productivity and energy reserves of Aedes mosquitoes from sewage drain habitats was made using Kolkata, West Bengal, India as a model geographical area. The ability of adult mosquitoes to transmit disease depends on the features of the individuals constituting the population. Life history traits serve as proxy markers to comment on the possible variations among individual mosquitoes and their disease transmission potential16. Thus, the present study included appraisal of the nutritional reserves and life history traits, along with the pupal productivity of Aedes in different larval habitats in Kolkata, India.
Material & Methods
The present study was conducted between January 2009 and December 2011 in the Entomology and Wildlife Biology Laboratory of the department of Zoology, University of Calcutta, Kolkata, India. Evaluation of the life history traits and nutritional reserves was performed under room temperature with a relative humidity of 70 per cent and above, mostly between June 2009 and February 2011.
Scheme for sampling Aedes breeding habitats: Entomological monitoring11 of the different containers of daily domestic usage and sewage drains as larval habitats of Aedes mosquitoes was carried out on monthly basis from selected sites of Kolkata during the three consecutive years (2009-2011). The swage drain water was analyzed with the help of a multi-parameter water analyzer (Multi-parameter PCSTestrTM 35; Eutech Instruments, Singapore; Oaklon®). At each site, the Aedes mosquito larval habitats were chosen randomly11 and 20 numbers of each habitat - earthen pots, porcelain and plastic containers, and sewage drains per month were considered.
Depending on the amount of water volume, the immature from the containers were collected either pulling with a pipette (if > 100 ml approximately)1 or transferring the whole content (if < 100 ml) in a separate specimen containers (Tarson® specimen container, India, 100 ml capacity)6. Sewage drains were sampled using short plankton net (7 or 9 cm diameter, 200 μm mesh size) moving horizontally and dredging through the bottom sediment as well. For all containers and sewage drains excess water was flushed and sieved with the help of plankton net (200 μm mesh size, rectangular in shape 30 × 15 cm) or using a circular plastic net (10 mm mesh size) to collect remaining immature, if any. The collected immature stages of Aedes mosquitoes were kept in the specimen containers with 75 ml water and covered with nylon net (10 mm mesh size), and brought to the laboratory for count and recording. Although the sampling design included all the possible habitats, the differences in proportional numbers of positive habitats could be a possible source of sampling error. Several other mosquitoes belonging to genera Culex, Anopheles, Armigeres, Lutzia, and Toxorynchites were encountered during sampling, but for the present study, only Ae. aegypti and Ae. albopictus were considered for analyses.
Laboratory parameters for assessment of the life history traits and nutritional reserves: Following collection of both larvae and pupae, and subsequent segregation, Aedes pupae were reared in plastic trays (15 × 11 × 3 cm) to obtain adults for evaluation of nutritional reserve. Individual pupa of Aedes spp. was weighed to record the pupal weight (wet weight up to nearest 0.1 mg using Mettlar-Toledo® Al-104 balance, Hong Kong), following which, each pupa was placed in individual vials, and allowed to emerge as adult. The sex and species of the adults were identified based on appropriate keys17. Following natural death of Aedes adults, the wing length (WL) of adults was measured to the nearest 0.1 mm using a dissecting stereo microscope (Olympus® SZX, Olympus Corporation, Tokyo, Japan) fitted with a graduated eyepiece (Erma®, Japan) (appropriate magnification, scaling and conversion of eyepiece to mm was followed). The adults were sacrificed for biochemical analysis for assessment of nutrient reserves such as amount of glycogen, sugar and lipid in Ae. aegypti and Ae. albopictus using single individual at a time1819.
Statistical analysis: To comment on the variations in the abundance of Aedes mosquitoes based on habitat types and months, the data on relative abundance of Aedes sp. were onsidered for three-way factorial ANOVA using month, species and habitat as variables20. The data on pupal weight and wing length of both the species and of either sex were also subjected to three-way factorial ANOVA, taking species, sex and habitat types as variables. For both Ae. aegypti and Ae. albopictus, correlation among life history traits and sex was estimated. The data obtained on the life history traits of Ae. aegypti and Ae. albopictus [i.e. pupal weight, PW (in mg), wing length, WL (in mm), amount of glycogen, GLY (in mg), amount of sugar, SUG (in mg), amount of lipid, LPD (in mg) in males and females] were used as explanatory variables for discriminating the habitats as dependent variables. The discriminant function analysis was performed to justify the habitat based differences in the life history traits of the mosquito species. DA would reflect the quality of sewage drain habitats against known habitats exploited by Aedes mosquitoes at a proximate level. The statistical analyses were performed following Zar20 using XLSTAT software21.
Results
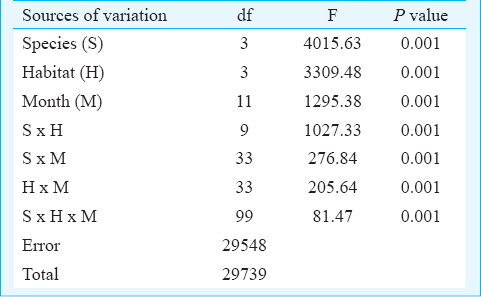
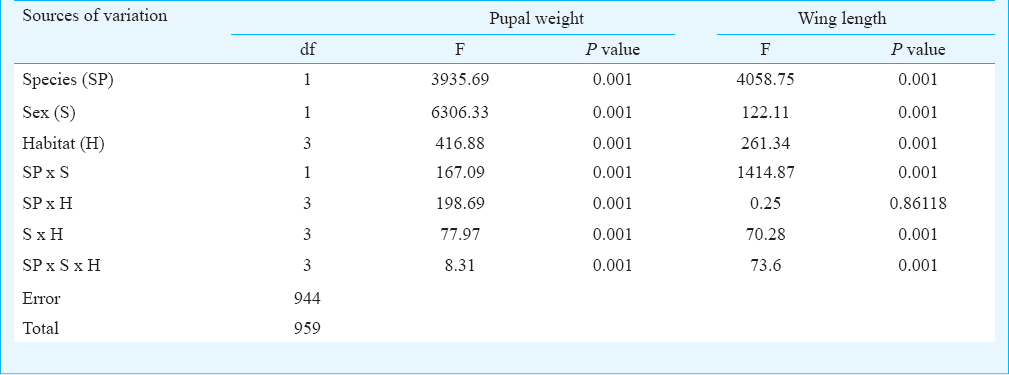
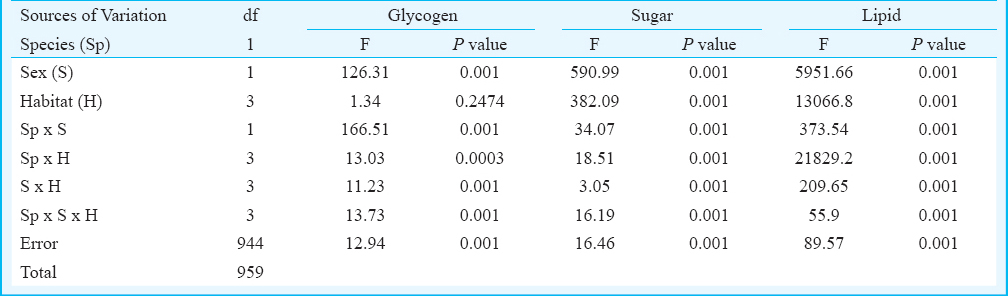
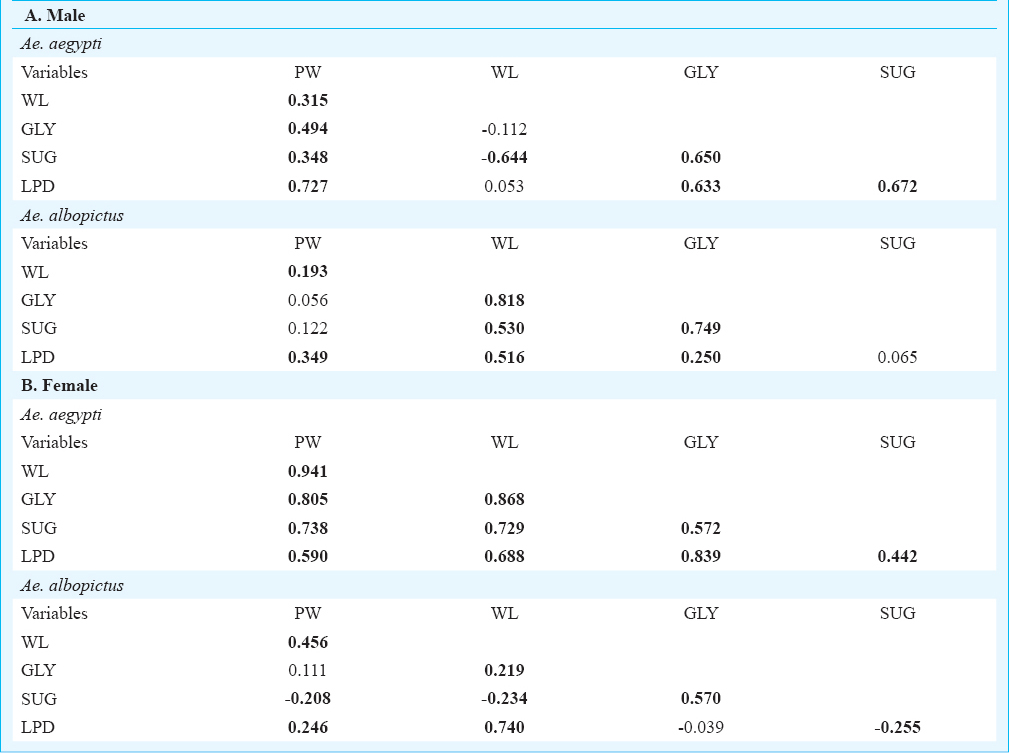
The number of individual Ae. aegypti and Ae. albopictus varied seasonally between the different habitats viz. various types of containers and sewage drains. The monsoon and post-monsoon months, from June to November, were noted to be most productive in sustaining the dengue vectors irrespective of the habitats. However, in sewage drains Ae. albopictus was observed more as compared to Ae. aegypti (Fig. 1). Analysis of the swage drain water indicated pH (7.78 ± 0.07), temperature (30.66 ± 0.97°C), electrical conductivity (2.754 ± 0.3 mS/cm), total dissolved solids (1.956 ± 0.23 ppt) and salinity (1.598 ± 0.18 ppt), similar to the gathered rainwater in mosquito larval habitats described elsewhere1415. The immature productivity was found to decrease in winter and summer seasons. The results of 3-way factorial ANOVA revealed that the abundance varied significantly (P<0.001) with the types of habitats, sampling months and species of the mosquitoes (Table I). The results of post-hoc Tukey test indicated significant (P<0.001) variations between all habitat pairs except between porcelain containers and sewage drain interaction. In both the species variations in the life history traits and energy reserves were prominent between the sexes and across the habitats (Fig. 2). Irrespective of habitats the mean pupal weight of males was 2.04 ± 0.25 mg (range 1.21 - 2.51 mg) while the mean weight of female pupae was 3.42 ± 0.25 mg (range 2.87 - 4.02 mg). As noted in the variation in the pupal weight, the mean wing length of male was 3.08 ± 0.21 mm (3.65 - 3.65 mm); compared to this, mean wing length of females was 33.44 ± 0.09 mm (3.28 - 3.67 mm). The glycogen and sugar contents of the males (0.12 ± 0.02 and 0.05 ± 0.01 mg, respectively) were also found to be lower than the females (0.18 ± 0.02 and 0.13 ± 0.007 mg, respectively). Similarly, for Ae. albopictus, males were lower in pupal weight (1.29 ± 0.05 mg; range 1.16 - 1.42 mg), and smaller in wing length (2.04 ± 0.12 mm; range 1.73 - 2.6 mm) than the females (pupal weight 2.29 ± 0.09 mg; range 2.07 - 2.46 mg; wing length 2.71 ± 0.09 mm, range 2.48 - 2.93 mm). The glycogen and sugar contents of the males (0.21 ± 0.04 and 0.15 ± 0.01 mg, respectively) were also found to be lower than the females (0.23 ± 0.03 mg and 0.20 ± 0.01 mg, respectively). Results of ANOVA on life history traits (pupal weight and wing length) and energy reserves (glycogen, sugar and lipid contents) revealed significant (P<0.001) variation between species, sex and types of habitats (Tables II and III). Species-specific variation and dependence of the pupal weight, wing length as well as the energy contents on the habitats was evident. However, interaction between the Aedes species (SP) and larval habitat (H) (Table II) and sex of the Aedes mosquitoes (Table III) did not impact the variation in wing length and glycogen content, respectively. The non-significance may be attributed to the independent variation of the variables. In all cases of pupal weight, wing length, glycogen, sugar and lipid content variation, the results of post-hoc Tukey test indicated significant differences between the habitat types. For both the sexes of either Ae. aegypti and Ae. albopictus, the different life history traits were correlated (Table IV).
![Variation in the mean abundance [log (x +1) transformed] of Aedes sp. in different habitat types observed in Kolkata, India during the study period (January 2009-December 2011). Values are mean ± SE (n=60).](/content/175/2015/142/Suppl 1/img/IJMR-142-87-g001.png)
- Variation in the mean abundance [log (x +1) transformed] of Aedes sp. in different habitat types observed in Kolkata, India during the study period (January 2009-December 2011). Values are mean ± SE (n=60).
![Habitat-based variation in different life history traits of Ae. aegypti (A) and Ae. albopictus (B) [pupal weight (PW in mg), wing length (WL in mm), glycogen (GLY in mg), sugar (SUG in mg) and lipid (LPD in mg)] content in individual mosquito. Values in each bar are mean ± SE (n=60).](/content/175/2015/142/Suppl 1/img/IJMR-142-87-g003.png)
- Habitat-based variation in different life history traits of Ae. aegypti (A) and Ae. albopictus (B) [pupal weight (PW in mg), wing length (WL in mm), glycogen (GLY in mg), sugar (SUG in mg) and lipid (LPD in mg)] content in individual mosquito. Values in each bar are mean ± SE (n=60).
Differences among the habitats were reflected in the biplot suggesting qualitative differences manifested in terms of Aedes life history traits (Fig. 3). Discriminant function analysis for Ae. aegypti indicated that the first two factors (F1 and F2) accounted for >99 per cent of variations of the data enabling discrimination among the four habitat types (sewage drains, earthen, plastic and porcelain containers) (Fig. 3A). The corresponding canonical correlations for F1 and F2 were 0.998 and 0.992, respectively for both the species. The same was apparent in Ae. albopictus, where factors F1 and F2 justified >99 per cent discrimination among the habitat (Fig. 3B). The corresponding canonical correlations for F1 and F2 were 0.914 and 0.758, respectively. For both the species, Fisher's distance varied significantly (P<0.05) among all the habitat pairs.
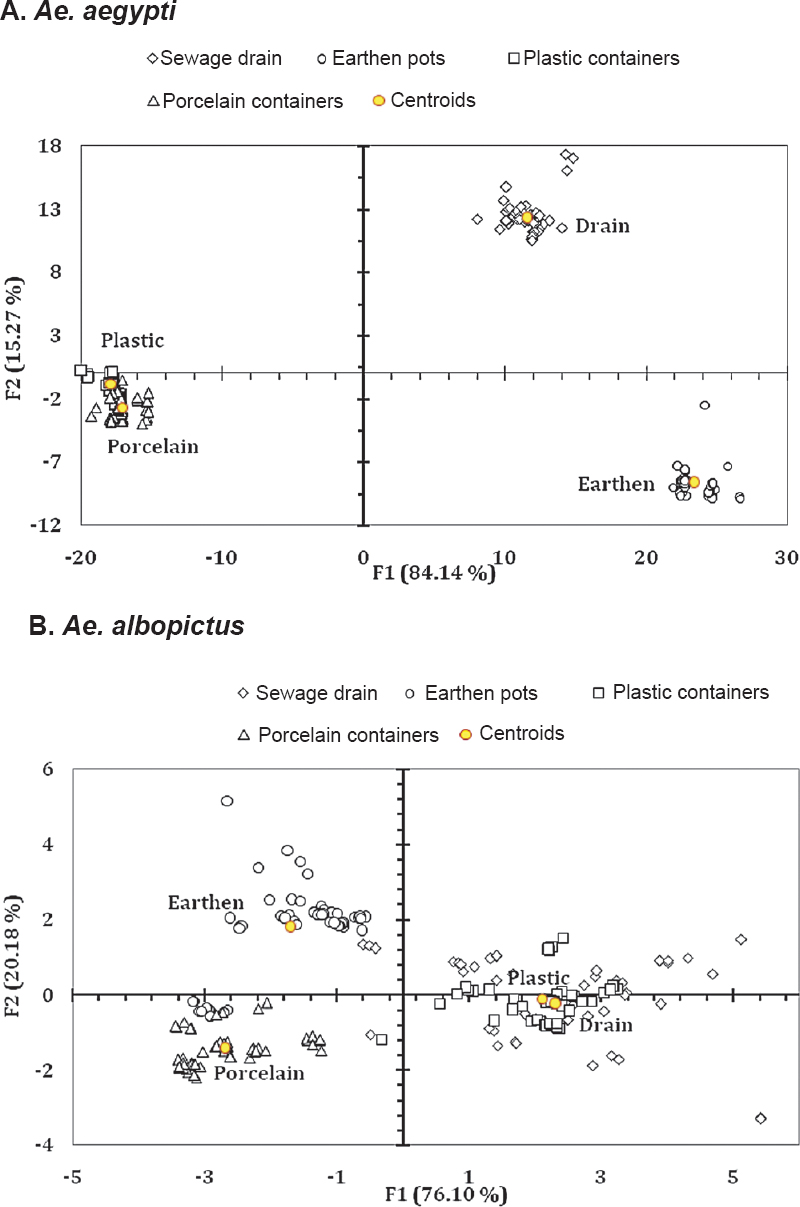
- Biplot representation of the ordination of the abundance of (A) Ae. aegypti and (B) Ae. albopictus in the different habitats surveyed as explained by the life history traits and energy reserves. For Ae. aegypti Wilks’ λ = 0.00002; F30, 667 = 948.054; P<0.001. For Ae. albopictus Wilks’ λ = 0.056; F30, 667 = 37.163; P<0.001.
Perhaps the water retention ability and resource availability of different habitats played a critical role in determining the trait values of dengue vectors. The sewage drain contributed towards production of adults having greater pupal weight and wing length and with higher values of energy reserves. Individuals of Ae. aegypti were noted to be heavier than Ae. albopictus and the females of both the species weighed more than the males. The same was true for wing length variation and nutrient reserve contents.
Discussion
The ability to breed in a wide variety of artificial containers facilitates the geographical range expansion and extensive spread of Ae. aegypti and Ae. albopictus101122. The quality of the larval habitats determines the pupal productivity and adult features of Aedes mosquitoes813, which in turn are linked to the disease transmission potential5716. Food resources in the larval habitats89, and competitions57 among developing individuals determine the adult size and thus fitness of Aedes mosquitoes. The numerical abundance of one species of Aedes over the other varies with the quality of the larval habitats2324. This was evident in the present study where Ae. albopictus was found in greater number in the sewage drains compared to Ae. aegypti. The size of the adults of both the mosquito species emerging from sewage drains was bigger than those emerging from rest of the larval habitats. Possibly the resource content of the sewage drains was higher than other habitats, which contributed to the development of large mosquitoes with higher nutrient reserves (indicated by pupal weight and wing length). Larval effort to acquire and assimilate energy is reflected through the corresponding pupal weight, nutrient reserve and adult body size2324. As a consequence, correlation between pupal weight, nutrient (glycogen, lipid and sugar) reserves, and wing length (body size) of mosquitoes was observed for both Ae. aegypti and Ae. albopictus in the present study. From disease transmission perspective, female Aedes mosquitoes with bigger body size than males will provide better biotope for the parasite and viruses to thrive. Inclusion of pupa weight and wing length5716 as additional parameters will enhance the existing entomological surveillance of dengue vectors1011.
In terms of abundance of immature of Aedes mosquitoes, the sewage drains were relatively less exploited compared to the other habitat types. The pupal weight, adult wing length and energy reserves indicated that sewage drains were equally congenial habitats as the established larval habitats like earthen, plastic and porcelain containers in the study area. Earlier studies2312 have shown that the availability of the containers is linked to household waste generation, and the subsequent pupal productivity in the containers varies with relative density and resources present for larval development892425. As sewage drains are not restricted in resource availability, these seem to be more favourable habitat for Aedes mosquitoes to thrive. However, container habitats with lower density of immature or higher nutrient reserve may provide equal opportunity to yield bigger mosquitoes. Our observations suggested that the sewage drains were utilized under conditions when the relative abundance of Aedes remained high (June to November) coinciding with monsoon and post-monsoon seasons. It is possible that addition of rainwater may have led to an overflow of water from the containers or reduce the organic matter content of the container habitats rendering them less effective as larval habitat. Alternatively, to avoid competition, and gain access to nutrients resources, breeding in water logged sewage drains was a better choice. Further monitoring of Aedes larval habitats including sewage drains can substantiate these observations.
Breeding of dengue vectors in unconventional habitats like sewage drains, underground water tanks4, and septic tank and cesspits131415, poses a concern for vector management. Following conventional strategy for Aedes control, if containers and smaller habitats are only targeted, as an alternative sewage drains and similar water bodies like cesspit and septic tanks may be utilized for breeding, resulting in comparatively fit individuals with higher reproductive success. From sewage drains, less number of individual Aedes mosquitoes with higher reproductive success may compensate for the loss due to check on container habitats. Continued water availability in sewage drains, septic tanks and cesspits make them permanent habitats for breeding in contrast to the container habitats, which are dependent on rain water or other sources. Although Aedes mosquitoes are known to occupy smaller habitats that are prone to drying, the risk to invasion in sewage drains and subsequent adaptation may increase the relative abundance in different geographical areas. Entomological monitoring for vector management may thus include sewage drains as habitat to reduce possibilities of breeding and sustenance of Aedes mosquitoes in the concerned geographical area.
Acknowledgment
The authors thank the respective Heads of the department of Zoology, University of Calcutta, Kolkata and The University of Burdwan, Burdwan for the facilities provided including DST-FIST. The first author (SB) acknowledges the financial assistance provided by the Council of Scientific & Industrial Research (CSIR), New Delhi, through Senior Research Fellow (SRF), in carrying out the work. The last author (GA) acknowledges the University Grants Commission (UCG), New Delhi for providing financial assistance through UGC Research Award.
Conflicts of Interest: None.
References
- Immatures of Aedes aegypti in Darjeeling Himalayas - expanding geographical limits in India. Indian J Med Res. 2009;129:455-7.
- [Google Scholar]
- Pupal productivity of dengue vectors in Kolkata, India: implications for vector management. Indian J Med Res. 2013;137:549-59.
- [Google Scholar]
- Household disposables as breeding habitats of dengue vectors: linking wastes and public health. Waste Manag. 2013;33:233-9.
- [Google Scholar]
- Presence of Aedes aegypti in water tanks on the ground and the implications for dengue control in Camagüey province. Rev Cubana Med Trop. 2010;62:93-7.
- [Google Scholar]
- Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc R Soc B Biol Sci. 2008;275:463-7.
- [Google Scholar]
- An assessment of macroinvertebrate assemblages in mosquito larval habitats - space and diversity relationship. Environ Monit Assess. 2010;168:597-611.
- [Google Scholar]
- Size alters susceptibility of vectors to dengue virus infection and dissemination. Am J Trop Med Hyg. 2008;79:688-95.
- [Google Scholar]
- Food as a limiting factor for Aedes aegypti in water storage containers. J Vector Ecol. 2004;29:11-20.
- [Google Scholar]
- Unusual life history traits of Aedes (Stegomyia mosquitoes (Diptera: Culicidae) inhabiting Nepenthes pitchers. Ann Entomol Soc Am. 2010;103:618-24.
- [Google Scholar]
- Eco-bio-social determinants of dengue vector breeding: a multicountry study in urban and periurban Asia. Bull World Health Organ. 2010;88:173-84.
- [Google Scholar]
- Multicountry study of Aedes aegypti pupal productivity survey methodology: findings and recommendations. TDR/IRM/DEN/06.1. Geneva: World Health Organization; 2006. p. :56.
- Seasonal prevalence of Aedes aegypti immatures in Kolkata, India. Southeast Asian J Trop Med Public Health. 2007;38:442-7.
- [Google Scholar]
- Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol. 2008;22:62-9.
- [Google Scholar]
- Septic tanks as larval habitats for the mosquitoes Aedes aegypti and Culex quinquefasciatus in Playa-Playita, Puerto Rico. Med Vet Entomol. 2010;24:117-23.
- [Google Scholar]
- Unusual developing sites of dengue vectors and potential epidemiological implications. Asian Pac J Trop Biomed. 2012;2:228-32.
- [Google Scholar]
- Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Trop Med Int Health. 2011;16:955-64.
- [Google Scholar]
- Phylogeny and classification of tribe Aedini (Diptera: Culicidae) Zool J Linn Soc. 2009;157:700-94.
- [Google Scholar]
- Rapid determination of glycogen and sugars in mosquitoes. J Am Mosq Control Assoc. 1985;1:299-301.
- [Google Scholar]
- Biostatistical analysis (4th ed). New Delhi, India: Pearson Education Singapore Pte. Ltd (Indian Branch); 1999. p. :663.
- Bamboo stumps as mosquito larval habitats in Darjeeling Himalayas, India - a spatial scale analysis. Insect Sci. 2008;15:245-9.
- [Google Scholar]
- A field test for competitive effects of Aedes albopictus on Ae. aegypti in South Florida: differences between sites of coexistence and exclusion? Oecologia. 2004;139:583-93.
- [Google Scholar]
- Detritus type alters the outcome of interspecific competition between Aedes aegypti and Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2008;45:375-83.
- [Google Scholar]
- Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Med Vet Entomol. 2000;14:31-7.
- [Google Scholar]






