Translate this page into:
Individualization of antiretroviral therapy - Pharmacogenomic aspect
Reprint requests: Dr Aruna Shankarkumar, Department of Transfusion Transmitted Disease, National Institute of Immunohaematology (ICMR), 13th floor, New Multistoreyed Building, KEM Hospital campus, Parel, Mumbai 400 012, Maharashtra, India e-mail: arp21@rediffmail.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Combination therapy with three drug regimens for human immunodeficiency virus (HIV) infection significantly suppresses the viral replication. However, this therapeutic impact is restricted by adverse drug events and response in terms of short and long term efficacy. There are multiple factors involved in different responses to antiretrovirals (ARVs) such as age, body weight, disease status, diet and heredity. Pharmacogenomics deals with individual genetic make-up and its role in drug efficacy and toxicity. In depth genetic research has provided evidence to predict the risk of developing certain toxicities for which personalized screening and surveillance protocols may be developed to prevent side effects. Here we describe the use of pharmacogenomics for optimal use of HAART (highly active antiretroviral therapy).
Keywords
HAART
HIV-NNRTI
NRTI
personalized medicine
pharmacogenomics
Introduction
Introduction of highly active antiretroviral therapy (HAART) has drastically reduced mortality associated with HIV infection, but variability in efficacy and toxicity is challenging. The advances in molecular biology has changed pharmacogenetics to a great extent to develop pharmacogenomics1. Host genetic factors are accountable for the variability in antiretroviral (ARV) response along with sex, body mass index, heredity and disease progression. Sometimes genetic variability is solely responsible for these variations23. A single-nucleotide change is a DNA sequence variation occurring commonly within a population at the same locus of the gene which may alter functioning of the protein product extensively. Single-nucleotide change hardly affects functioning of the protein produced by that gene, but some changes affect functioning. Single nucleotide polymorphisms (SNPs) are significant with respect to genes of drug metabolic pathway including enzymes, drug carrier proteins involved in pharmacokinetics and disease progression. The influence of SNPs on personal responses to pharmacotherapy is complicated. Genetic variations in pathways of drug absorption, disposition, metabolism and excretion (ADME) contribute to inter-patient differences. Therefore, genes encoding for transport proteins, drug metabolizing enzymes or nuclear receptors have been the main targets of HIV pharmacogenomic studies. So far, numerous associations of SNPs with susceptibility to ARV drug adverse reactions or risks of virological failure have been reported4. This review focuses on some important aspects of pharmacogenomics to maximize efficacy and minimize toxic effects of HAART.
Pharmacogenomics
Combination antiretroviral therapy (cART) for HIV - HAART or cART has improved the prognosis of HIV infection close to normal life expectancy. With HAART, different combinations of antiretroviral drugs are available for untreated and treated individuals. There are six categories of drug regimens available as follows: (1) Nucleoside reverse transcriptase inhibitors (NRTIs), (2) Non-nucleoside reverse transcriptase inhibitors (NNRTIs), (3) Protease inhibitors (PIs), (4) Integrase inhibitors (IIs), (5) Fusion inhibitors (FIs), and (6) Chemokine receptor antagonists. The choice of treatment is mainly influenced by HIV disease stage, co-morbidities, co-medication with potential drug-drug interactions, pregnancy or pregnancy potential, expected cART toxicity, and results of genotypic resistance testing and synergism/antagonism of the combination on HIV virus life cycle. Table I gives summary of some key reported genetic variants for HAART. Table II provides information on pharmacogenetics of adverse reactions due to HAART.
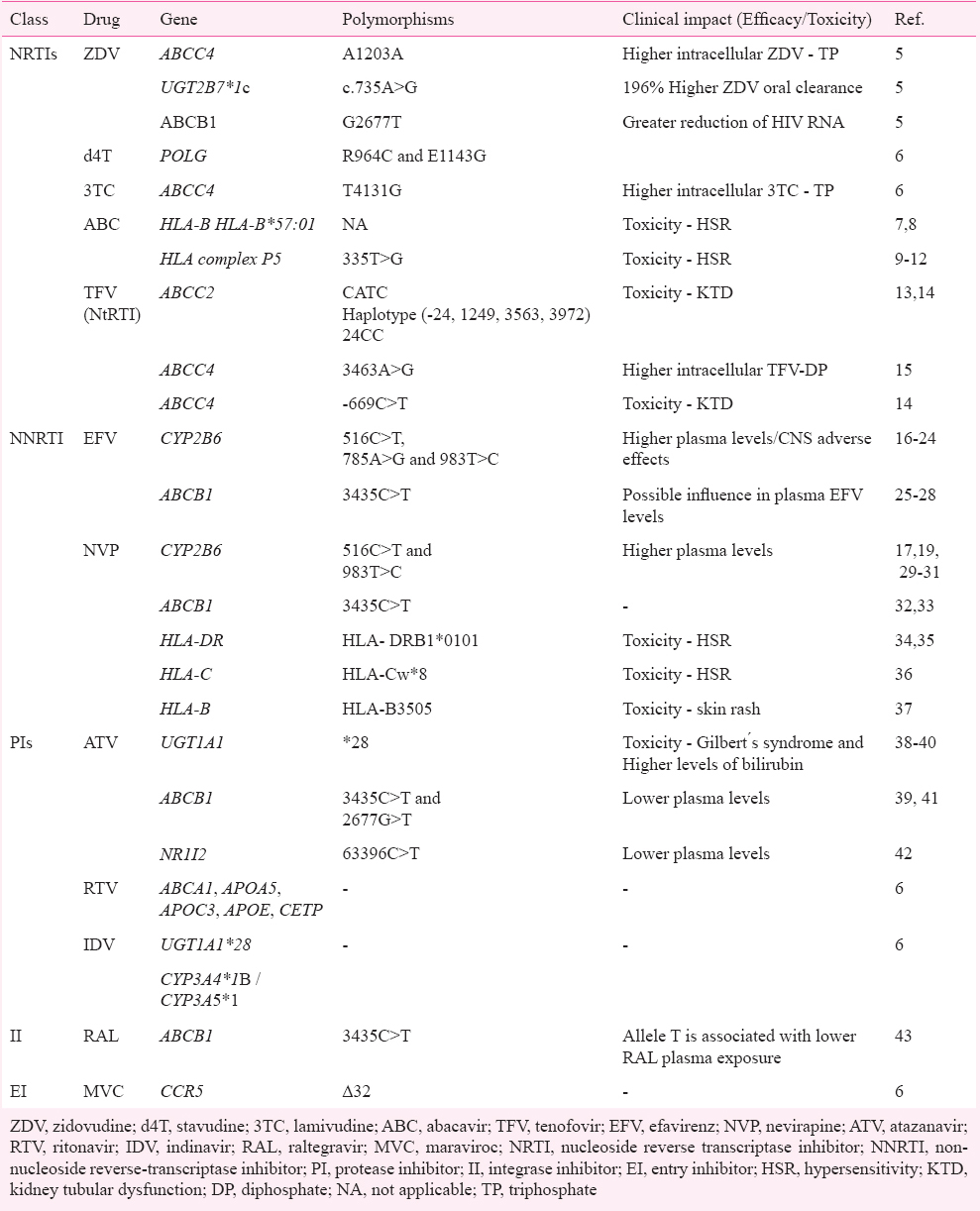
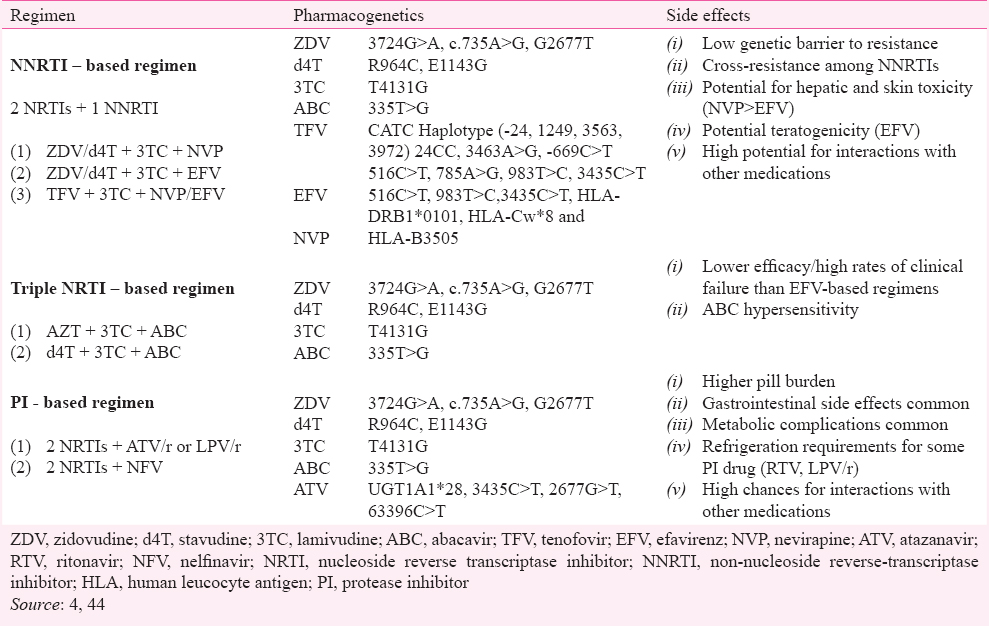
1. Nucleoside reverse transcriptase inhibitors (NRTIs): NRTI is a nucleoside analogue antiretroviral drug and its chemical structure constitutes a modified version of a natural nucleoside. These inhibit viral replication of retroviruses by stopping extension of oligomer due to absence of 3’ hydroxyl group essential for addition of incoming new nucleotide. This prevents further synthesis of viral nucleic acid by interfering with the reverse transcriptase enzyme45 (Fig. 1). These drugs get activated on endocytosis after phosphorylation to form active triphosphate compound (pro-drugs). The known drug toxicities are linked with lipid metabolism, liver steatosis and lactic acidosis. Additional evidence relates NRTI drugs to disruption of mitochondrial function, oxidative stress and peripheral neuropathy46.
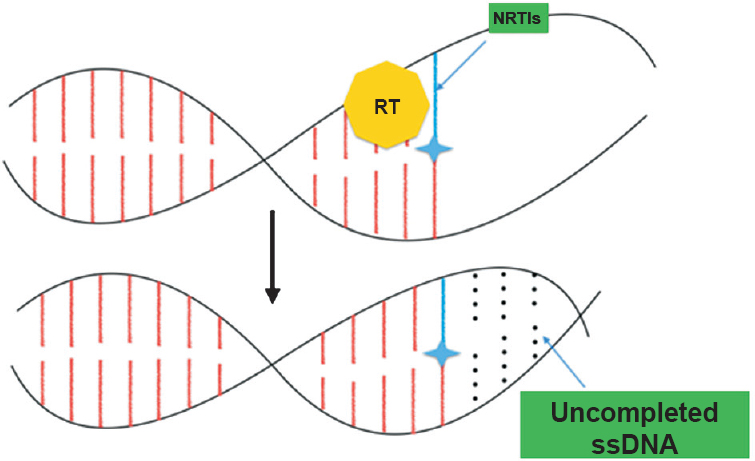
- Mode of action of nucleoside reverse transcriptase inhibitor (NRTI). On incorporation of NRTI the chain is terminated. ssDNA, single strand DNA.
Zidovudine (ZDV) (Azidothymidine, AZT) - Thymidine analogue - ZDV has been prescribed after U.S. Food and Drug Administration (FDA) approval in 1987 as a part of drug regimen to treat HIV infection, till it was found more harmful than tenofovir47. ZDV was found to be associated with severe anaemia in a randomized trial in untreated HIV patients using efavirenz (EFV) in addition to ZDV + lamivudine (3TC) / tenofovir (TDF) + emtricitabine (FTC) where ZDV was removed in 5.5 per cent population under study48. A previous study showed that 16.2 per cent of patients developed ZDV induced anaemia in the population of eastern India49, 58 per cent of the patients on ZDV showed reduced viral load (<400 copies/ml) as compared to 71 per cent cases on TDF only because ZDV had to be withdrawn in 11 per cent (vs 5%) of the cases after drug adversities in 144 wk49. Observed ZDV related toxicities include drop in haemoglobin level and neutrophils count, and nausea due to gastrointestinal disturbances within a few weeks on initiation of the trial. Also, fat deposition in upper and lower extremities was significantly reduced on ZDV treatment50.
A pharmacogenetic study has reported higher levels of ZDV-triphosphates (up to 49%) in HIV cases heterozygotes for ABCC4 G3724A on ZDV treatment as compared to wild type GG homozygote51. Another variant ABCB1 GT or TT has shown significantly reduced levels of HIV viral load than that in individuals having wild type GG genotype52. Kwara et al53 have shown 196 per cent oral drug clearance associated with UGT2B7*1c carriers on ZDV therapy when compared to non carriers. These studies emphasize on extensive analysis using large cohorts for understanding potential role of pharmacogenomic factors in ZDV pharmacokinetics and pharmacodynamics5.
Stavudine (d4T) - Thymidine analogue - Stavudine (2’,3’-didehydro-2’,3’-dideoxythymidine) was made available for patients showing virological failure or intolerance to ZDV after its approval in May 199654. After one year, it became the preferred drug for ART. But it was found to be associated with susceptibility to body fat side effects. Therefore, in 2009, the World Health Organization (WHO) restricted its use due to long-term, irreversible side effects55.
Lamivudine (3TC) - Cytidine analogue - Lamivudine (2’,3’-dideoxy-3’-thiacytidine) is another nucleotide analogue used in combination therapy for delaying developments of acquired immunodeficiency syndrome (AIDS) and hence, avoiding disease related complications or cancer. Higher intracellular concentrations of 3TC-triphosphate have been reported to be associated with multidrug resistance protein 4 (MRP4) T4131G change and MRP2 polymorphism49.
Tenofovir disoproxil fumarate (TDF) - Adenosine analogue - Tenofovir, one of the most effective and commonly prescribed antiretroviral drugs belongs to a class of nucleotide reverse transcriptase inhibitors (NtRTIs). Nucleotide analogues have phosphate group in addition to pentose and nucleic base of nucleoside. Unlike nucleoside analogues, NtRTIs are chemically preactivated and thus require less processing in the body. A single G>A substitution at 1249 nucleotide of ATP-binding cassette, sub-family C, member 2 (ABCC2) gene is reportedly linked with TDF adverse reaction causing renal tubulopathy6. Interestingly, TDF is not a substrate for MRP2, but for MRP415. More recently, a naïve association between ABCC4 3463A>G genotype and renal toxicity has been reported showing tenofovir concentrations 35 per cent higher in carriers of the 3463G variant44.
Abacavir (ABC) - Guanosine analogue - Hypersensitivity to ABC occurs in about five per cent of HIV infected patients usually by the second week of ABC treatment. In some cases, hypersensitivity reaction is seen by six weeks56. A polymorphism in HLA gene HLA-B*5701 needs to be screened prior to initiation of ABC treatment to avoid hypersensitivity reaction. An inexpensive laboratory test is available to detect this gene57. HLA B57 frequency is 5-20 per cent in India58. An Indian study58 demonstrated that HLA B17 frequency in HIV patients on antiretroviral therapy was due to the different composite ethnic groups studied. The testing for HLA B17 antigen along with HLA B*5701 allele subtype can be used as pharmacogenetic testing to prevent abacavir hypersensitivity reaction among Indian patients58. This testing is now mandatory in many countries before prescribing abacavir9. HCP5 335 T>G polymorphism in P5 gene of HLA is preferred SNP marker over HLA B*5701 for ABC sensitivity due to simplicity and economicity101112.
2. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): Non-nucleosides are directly active drugs as against prodrug NRTIs. These prevent HIV replication by targeting the enzyme reverse transcriptase, binding to a site near to but different from the active site for substrate. It decreases DNA synthesis drastically (Fig. 2). ADME pathways genes are studied extensively focusing on CYP450 enzyme4. Detoxification of NNRTI takes place in liver by CYP450 enzyme. SNPs associated with these isoenzymes play crucial role in inter-individual differences in metabolism and disposition of NNRTIs59. Polymorphisms result in decreased expression of enzymes and their activity in liver microsomes. As a result, there is considerable inter-individual variability in NNRTI ADME. Also, genes encoding drug transporters and HLA are considered in the pharmacogenetic studies of NNRTI. SNPs in MDR1 gene, which encodes for P-glycoprotein (P-gp) affect oral absorption and desorption of NNRTIs. P-glycoprotein acts as a NNRTI carrier. The association between variants of MDR1 gene and NNRTI plasma concentrations has been studied comprehensively44.
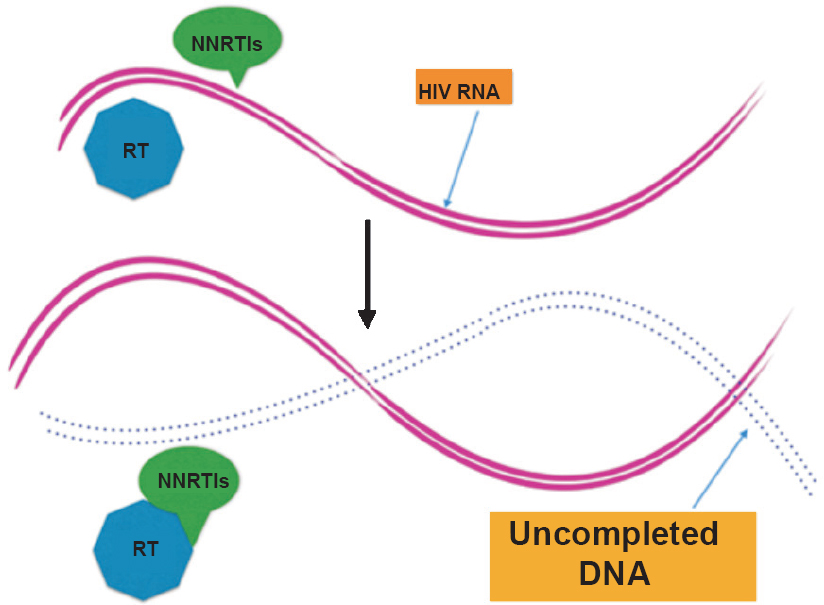
- Mode of action of non-nucleoside reverse transcriptase inhibitor (NNRTI). NNRTIs block enzyme activity by binding directly to reverse transcriptase (RT) enzyme.
Nevirapine (NVP) - In 1997, NVP was approved as NNRTI60. In developing countries, it is the preferred first line drug in combination with two NRTIs due to its efficacy, moderate price and adjustable dosage60. NVP causes elevation of liver enzymes which may occasionally be severe. About 15-20 per cent patients experience rash and NVP needs to be withdrawn in about 7 per cent of them61. NVP-induced rashes were reported in 2.14 per cent of HIV positive individuals from India62. It is noteworthy that liver damage may appear after many months63. NVP induced hypersensitivity is associated with HLA-DRB1 allele and MDR1 gene polymorphism323464. The polymorphism ABCB1-3435C>T is linked with a decreased risk of hepatotoxicity in patients receiving NVP2530. NVP plasma concentration is affected by G516T and 983 T>C substitutions in CYP2B6 gene. Also, significantly higher NVP plasma levels are reported in black patients heterozygous for T983C SNP1617. Although sex, age, body mass index, habits, habitat and pathological liver condition are major criteria in influencing pharmacokinetics of NVP, but in most of the studies only body weight is included294457.
Efavirenz (EFV) - EFV is the first line drug for untreated HIV infected cases. EFV shows a narrow therapeutic range and there is a potential risk of high level of therapeutic concentrations that are related with virological failure or neurological manifestations. It is metabolized by CYP2B6 in liver. The G516T SNP in CYP2B6 (CYP2B6*6) is reported to have a major impact on the pharmacokinetics and pharmacodynamics of EFV65. Black patients are predisposed to the CNS effects due to high prevalence of the genotype CYP2B6 - 516TT1819. Also, there is SNP variability due to 785 A>G, 983 T>C, 593 T>C and 1132 C>T substitutions in CYP2B6 rendering slow metabolization of EFV65. This affects its pharmacokinetics with increased drug levels, which result in CNS events or virological failure, necessitating CYP2B6 allele genotyping. Efavirenz disrupts sleep architecture66.
3. Protease inhibitors (PIs): HIV hijacks host genetic code on invading CD4 cell and utilizes host cell machinery for its replication. The viral gag-pol polyprotein is excised into active protein particles of newly formed virus by viral protease, a molecular scissor. This step is inhibited by blocking the protease enzyme using PIs by preventing a proteolytic splicing and results into non-infectious virus particles (Fig. 3). Toxic effects of PIs include lipodystrophy, dyslipidaemia, tolerability problems and gastrointestinal disturbances67. Often drug interactions are substantiated due to PIs68. PIs are mostly metabolized by CYP3A4. PIs act both as inhibitors of and substrate for CYP3A4. CYP3A4 also metabolizes both simvastatin and lovastatin. When PIs act as inhibitor of CYP3A4, the levels of simvastatin and lovastatin may increase drastically6970. This in turn increases the risk of toxicity of liver and skeletal muscle71.
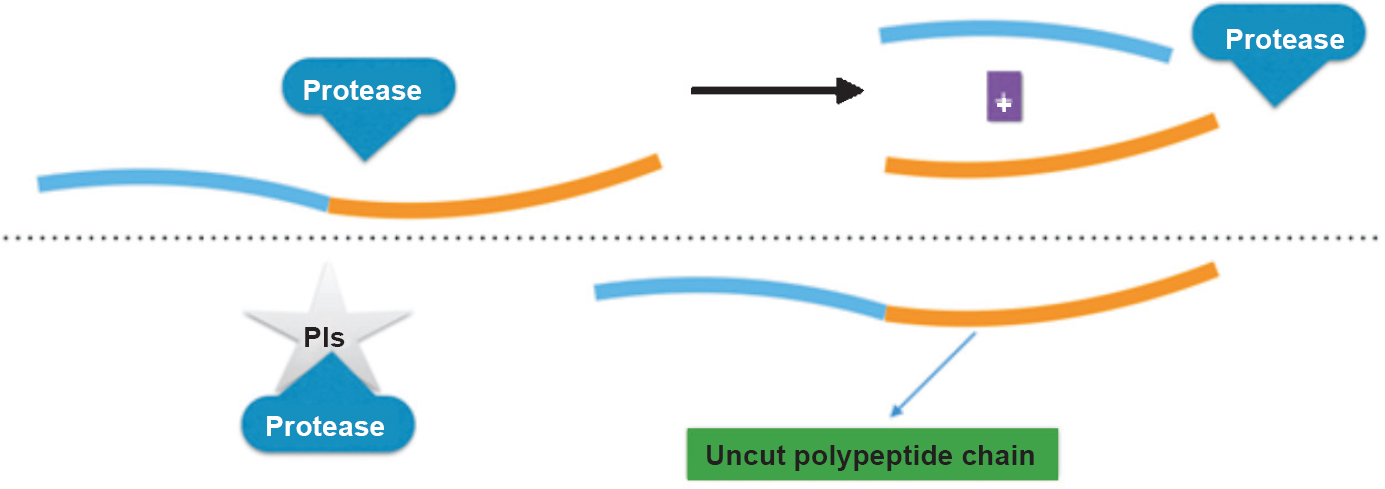
- Mode of action of protease inhibitors (PIs). PIs are substrate analogue (mimic protein cutting site), and prevent generation of new viral proteins.
Ritonavir (RTV) - Ritonavir is included in combinational regimen because it blocks host enzymes involved in metabolism of PIs. It is the most powerful of all the PIs even in low doses. The primary role of RTV in boosted PI regimens is to improve the pharmacokinetics of the second PI. RTV inhibits host enzymes of drug metabolic pathways causing their levels to rise in bloodstream, and increasing efficacy of other PIs with reduced dose and frequency rendering them more compliant. It also blocks its own metabolism by inhibiting cytochrome P45072. Liver enzymes metabolize protease inhibitors only when their activity is modified. All PIs decrease hepatic enzyme activity. These increase cholesterol and triglycerides levels, cause abnormal fat deposition in various parts of the body, and diabetes. These can be associated with polymorphisms of genes like ATP-binding cassette transporter A1 (ABCA1), apolipoprotein A5 (APOA5), apo lipoprotein C3 (APOC3), apo lipoprotein E (APOE), and cholesterol ester transfer protein (CETP)73. APOE and APOC3 variant alleles are associated with lipodystrophy among HIV patients. Genetic screening can reduce risk of hypertriglyceridaemia associated with RTV7475.
Atazanavir (ATV) - In March 2004, ATV arrived in market as daily prescription in single dose. Increased lipid levels associated with this drug make oneself susceptible to cardiovascular event74. Fifty per cent of cases on ATV show rise in bilirubin levels and above one third have grade 3-4. This is also true for lipodystrophy7576. Some patients even develop jaundice as hepatic conjugation is hampered due to a mechanism similar to Gilbert's Syndrome. A genetic predisposition is identified77. This side effect is provoked by competitive inhibition of UDP glucuronosyl transferase 1 family polypeptide A1 (UGT1A1), the microsomal enzyme causing glucuronidation, which allows bilirubin excretion. Inconsequential rise needs withdrawal of ATV in a few patients due to overt jaundice78. A previous Indian study has reported increased prevalence of of (TA)7/(TA)7 genotype in neonates (allele frequency 0.366)38. UGT1A1 promoter variant is associated with hyperbilirubinaemia79. The presence of SNP UGT1A1*28 is strongly associated with the occurrence of jaundice because of decreased enzyme activity525780.
Indinavir (IDV) - Highly penetrating IDV reaches genital compartments and CNS, but it is not preferred nowadays39. This medicine needs to be used with caution in India due to presence of extensive ‘renal stone belt’ to avoid the risk of nephrolithiasis8182.
4. Integrase inhibitors (IIs): HIV integrase is an important enzyme in its replication through integrating viral DNA with host genome83. Integrase inhibitor is a desirable anti-HIV drug as integrase enzyme is absent in human cells; its selective inhibition without side effects is possible (Fig. 4).
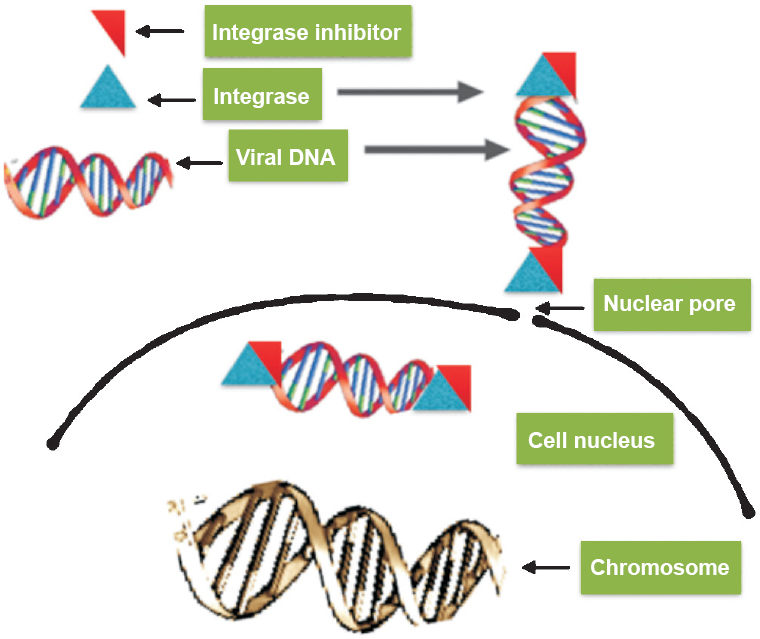
- Integrase inhibitors (IIs) - mode of action. IIs inhibit enzyme activity (bind to enzyme) and prevent insertion of proviral DNA.
Raltegravir (RAL) - Raltegravir is an aphthyridine carboxamide derivative that inhibits integrase. Integrase catalyses the step-wise process of integrating HIV-1 DNA into the host genome. In the process, assembly of integrase with viral DNA forms stable pre-integration complex, which follows endonucleolytic processing of the viral DNA ends, and joining of the viral and host DNAs. Covalent bonding takes place in 5’ phosphate groups exposed on nicking cellular DNA strands of host and viral DNA to produce provirus. RAL does not allow pre-integration complex to bind to host DNA84. It prevents replication of HIV-2 and acts against R5 and X4 tropic viruses. RAL is a substrate of the P-gp, and consequently, polymorphisms in transport proteins may explain the large intra- and inter-individual variations of RAL exposure8586. A recent study in Spanish HIV RAL-cohort reported that the polymorphism at ABCB1-3435C>T was associated with RAL concentrations43. Patients carrying CT or TT genotypes displayed lower median RAL concentrations than those with the CC genotype. Although pharmacokinetic/pharmacodynamics (PK/PD) analyses do not suggest a threshold RAL concentration associated with reduced efficacy, patients carrying CT/TT genotypes at the P-gp gene might be more prone to virological failure443. UGT1A1*28/*28 genotype is associated with higher RAL plasma concentrations compared to that of UGT1A1*1/*1 genotype8788.
5. Fusion inhibitors (FI): Thirty-six amino acids long peptide of the FI enfuvirtide (T-20) is similar to a portion of gp41, necessary for binding to heptad repeat 1 (HR1). It interferes with HR1 and HR2 interaction and thereby, inhibiting the conformational change crucial for viral fusion to CD4 cells. Decreased efficacy of T-20 is associated with a single amino acid substitution in HR157. The drug is efficacious with hardly any side effects. However, its high cost, intolerance on long term use, complex mode of administration and limited pharmacokinetic properties are the limiting factors, and in future, studies should focus on these aspect to enhance its clinical advantage89.
6. Entry inhibitors (EI): HIV enters human body through fusion of viral envelope proteins with binding domain on host CD4 + (cluster of differentiation 4) cell. EIs interfere with this fusion step and stop virus from entering into the cell, thereby restricting HIV from infecting a cell and multiplying. Theoretically inhibitors can interfere with every step of HIV entry into the host cell. EIs mainly target either the viral envelope glycoprotein gp120 or gp41 or the C-C chemokine receptor CCR5 or CXCR4 receptors on a CD4 cell surface90. Intracellular HIV inhibition is not possible by EIs as against other drug classes.
Maraviroc (MVC) - Maraviroc belongs to entry inhibitors called as CCR5 receptor antagonist. HIV makes entry into host cell using a CCR5 receptor present on CD4+ immune cells. MVC prevents entry of HIV by inhibiting its fusion with the CCR5 receptors (Fig. 5). It allosterically binds to CCR5 by inducing conformational changes within CCR5 and hence, inhibiting its binding to viral gp12091. It acts against CCR5 tropic viral strains but is inactive against CXCR4 tropic HIV strains. CCR5-Δ32 is an allele of CCR5. An allele Δccr5 of the β-chemokine receptor gene CCR5 has been found to confer protection against HIV-192. In Europeans the prevalence of this allele is 5-14 per cent but is uncommon in Africans and Asians93. This protective allele was found to be absent in majority of Indians. Its sporadic occurrence in southern and northern parts of India presume Caucasian admixture92. The HIV R5 entry is prevented because of Δ32 deletion leading to a non-functional receptor. Homozygosity for this allele gives strong protection against HIV infection whereas the heterozygosity is associated with milder disease progression94. However, genetic variations in the CCR5 gene have not been shown to affect virological response to MVC95. On the other hand, MVC is substrate of CYP3A4 and P-gp; hence, dose adjustment is frequently required when co-administered with drugs that alter its pharmacokinetics9697. MVC is identified as substrate of the transport protein OATP1B1 and the variation 521T>C is linked with higher MVC plasma levels98.Thus, the only genetic test that is mandatory to perform before starting MVC treatment is the determination of viral tropism499.
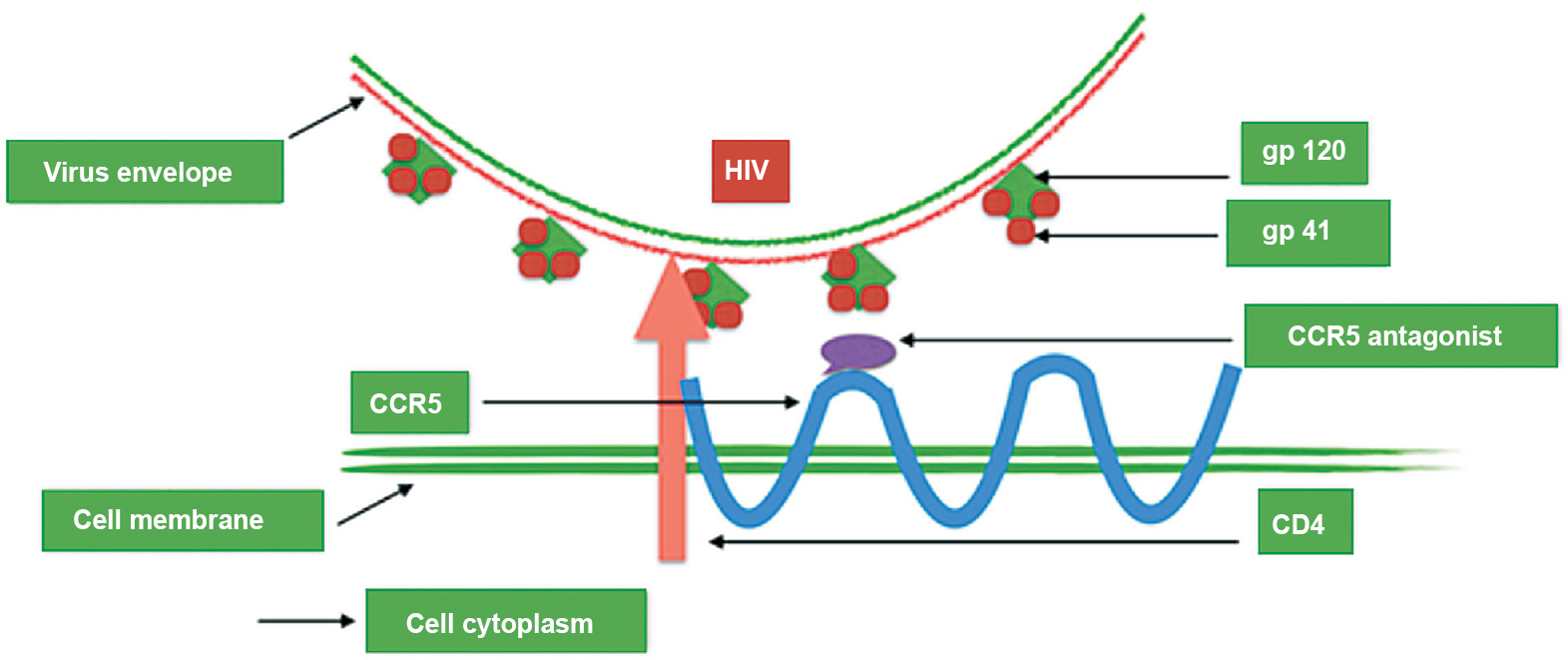
- Mode of action of maraviroc. It prevents gp120 from binding to the CCR5 co-receptor and prevents the fusion peptide from gp41 inserting into the cell membrane.
Conclusion and clinical relevance
The objective of any therapy is to maximize the therapeutic outcome and to minimize the side effects. HAART represents very effective ART but is brought with innumerable side effects. The side effects like cutaneous hypersensitivity may be avoided with proper genetic testing which predisposes to susceptibility such as HLA-B*5701 associated ABC adverse reaction. Similarly, certain drugs like maraviroc may not be prescribed to HIV positive individuals with CCR5 tropic HIV. However, a large number of HAART drugs show change in pharmacokinetics due to variations in genes of drug metabolic pathway enzymes like CYP450, ATP binding cassette and UGTA1. Individualized approach to personalized HAART is influenced by host factors and is known as pharmacogenomics. It is also influenced by certain viral characteristics and the drug administered. Application of pharmacogenomics with understanding of medicine, viral characteristic of HIV is at the doorstep to guide the personalized prescription in which the right medication will be given to the right person. Several measures need to be taken for application of pharmacogenetic research in making a genetic test available for public. Many factors affect successful translation such as pharmacodynamics and pharmacokinetic properties of drug adversities, laboratory facilities, quality assurance and quality control (QA and QC) measures and type of tests100. The reported associations have to undergo stringent validation in independent, ethnically diverse populations having highest number of HIV positive individuals.
References
- Pharmacogenetics, pharmacogenomics, and individualized medicine. Pharmacol Rev. 2011;63:437-59.
- [Google Scholar]
- Hypothesis: comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics. 1998;8:283-9.
- [Google Scholar]
- The pharmacogenetics of HIV treatment: A practical clinical approach. J Pharmacogenomics Pharmacoproteomics. 2013;4:116.
- [Google Scholar]
- Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71:619-27.
- [Google Scholar]
- Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727-32.
- [Google Scholar]
- Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121-2.
- [Google Scholar]
- Association of the genetic marker for abacavir hypersensitivity HLA-B*5701 with HCP5 rs2395029 in Mexican Mestizos. Pharmacogenomics. 2011;12:809-14.
- [Google Scholar]
- The HCP5 single-nucleotide polymorphism: a simple screening tool for prediction of hypersensitivity reaction to abacavir. J Infect Dis. 2008;198:864-7.
- [Google Scholar]
- Use of the HCP5 single nucleotide polymorphism to predict hypersensitivity reactions to abacavir: correlation with HLA-B*5701. J Antimicrob Chemother. 2010;65:1567-9.
- [Google Scholar]
- Rapid HCP5 single-nucleotide polymorphism genotyping: a simple allele-specific PCR method for prediction of hypersensitivity reaction to Abacavir. Clin Chim Acta. 2011;412:1382-4.
- [Google Scholar]
- Predictors of kidney tubular dysfunction in HIV-infected patients treated with tenofovir: a pharmacogenetic study. Clin Infect Dis. 2009;48:e108-16.
- [Google Scholar]
- Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J Infect Dis. 2006;194:1481-91.
- [Google Scholar]
- Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47:298-303.
- [Google Scholar]
- Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an Adult AIDS Clinical Trials Group Study. Clin Infect Dis. 2006;42:401-7.
- [Google Scholar]
- Influence of CYP2B6 polymorphism on plasma and intracellular concentrations and toxicity of efavirenz and nevirapine in HIV-infected patients. Pharmacogenet Genomics. 2005;15:1-5.
- [Google Scholar]
- Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18:2391-400.
- [Google Scholar]
- German Competence Network for HIV/AIDS. Impact of CYP2B6 983T>C polymorphism on non-nucleoside reverse transcriptase inhibitor plasma concentrations in HIV-infected patients. J Antimicrob Chemother. 2008;61:914-8.
- [Google Scholar]
- Influence of 516G>T polymorphisms at the gene encoding the CYP450-2B6 isoenzyme on efavirenz plasma concentrations in HIV-infected subjects. Clin Infect Dis. 2005;40:1358-61.
- [Google Scholar]
- Effect of CYP2B6, ABCB1, and CYP3A5 polymorphisms on efavirenz pharmacokinetics and treatment response: an AIDS Clinical Trials Group Study. J Infect Dis. 2010;202:717-22.
- [Google Scholar]
- Homozygous CYP2B6 *6 (Q172H and K262R) correlates with high plasma efavirenz concentrations in HIV-1 patients treated with standard efavirenz- containing regimens. Biochem Biophys Res Commun. 2004;319:1322-6.
- [Google Scholar]
- Genetic variability of CYP2B6 in populations of African and Asian origin: allele frequencies, novel functional variants, and possible implications for anti-HIV therapy with efavirenz. Pharmacogenet Genomics. 2005;15:861-73.
- [Google Scholar]
- Identification of a novel specific CYP2B6 allele in Africans causing impaired metabolism of the HIV drug efavirenz. Pharmacogenet Genomics. 2006;16:191-8.
- [Google Scholar]
- Associations between ABCB1, CYP2A6, CYP2B6, CYP2D6, and CYP3A5 alleles in relation to efavirenz and nevirapine pharmacokinetics in HIV-infected individuals. Ther Drug Monit. 2012;34:153-9.
- [Google Scholar]
- Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359:30-6.
- [Google Scholar]
- No influence of the P-glycoprotein genotype (MDR1 C3435T) on plasma levels of lopinavir and efavirenz during antiretroviral treatment. Eur J Med Res. 2003;8:531-4.
- [Google Scholar]
- Population pharmacokinetic/pharmacogenetic model for optimization of efavirenz therapy in Caucasian HIV-infected patients. Antimicrob Agents Chemother. 2011;55:5314-24.
- [Google Scholar]
- Integration of population pharmacokinetics and pharmacogenetics: an aid to optimal nevirapine dose selection in HIV-infected individuals. J Antimicrob Chemother. 2011;66:1332-9.
- [Google Scholar]
- Toxicogenomics of nevirapine-associated cutaneous and hepatic adverse events among populations of African, Asian, and European descent. AIDS. 2011;19:1271-80.
- [Google Scholar]
- Characterization of the in vitro biotransformation of the HIV-1 reverse transcriptase inhibitor nevirapine by human hepatic cytochromes P-450. Drug Metab Dispos. 1999;27:1488-95.
- [Google Scholar]
- Drug transporter and metabolizing enzyme gene variants and nonnucleoside reverse-transcriptase inhibitor hepatotoxicity. Clin Infect Dis. 2006;43:779-82.
- [Google Scholar]
- Nevirapine-induced hepatotoxicity and pharmacogenetics: a retrospective study in a population from Mozambique. Pharmacogenomics. 2010;11:23-31.
- [Google Scholar]
- Predisposition to nevirapine hypersensitivity associated with HLA-DRB1*0101 and abrogated by low CD4 T-cell counts. AIDS. 2005;19:97-9.
- [Google Scholar]
- HLA- DRB1*01 associated with cutaneous hypersensitivity induced by nevirapine and efavirenz. AIDS. 2008;22:540-1.
- [Google Scholar]
- HLA-dependent hypersensitivity to nevirapine in Sardinian HIV patients. AIDS. 2006;20:1621-6.
- [Google Scholar]
- HLA-B*505 allele is a strong predictor for nevirapine-induced skin adverse drug reactions in HIV-infected Thai patients. Pharmacogenet Genomics. 2009;19:139-46.
- [Google Scholar]
- Prevalence of clinically relevant (TA)n UGT1A1 promoter alleles in Indian neonates. Curr Sci. 2013;105:446-7.
- [Google Scholar]
- Discrepancies between protease inhibitor concentrations and viral load in reservoirs and sanctuary sites in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2003;47:238-3.
- [Google Scholar]
- Genome-wide association study of atazanavir (ATV) pharmacokinetics (PK) and indirect hyperbilirubinemia (HBR). Abstract No. J-165. In: Proceedings of the 20th Conference on retroviruses and opportunistic infections. 2013.
- [Google Scholar]
- Plasma levels of atazanavir and the risk of hyperbilirubinemia are predicted by the 3435C-->T polymorphism at the multidrug resistance gene 1. Clin Infect Dis. 2006;42:291-5.
- [Google Scholar]
- Association of a single-nucleotide polymorphism in the pregnane X receptor (PXR 63396C-->T) with reduced concentrations of unboosted atazanavir. Clin Infect Dis. 2008;47:1222-5.
- [Google Scholar]
- Polymorphisms in the ABCB1 gene (P-glycoprotein) influences raltegravir concentration. In: Proceedings of the 6th IAS Conference on HIV pathogenesis, treatment and prevention. 2011. Abstract No. MDPE200
- [Google Scholar]
- Human immunodeficiency virus: pharmacogenetics of antiretroviral treatment. J Pharmacogenomics Pharmacoproteomics. 2011;S6:002.
- [Google Scholar]
- HIV-1 RT inhibitors with a novel mechanism of action: NNRTIs that compete with the nucleotide substrate. Viruses. 2010;2:880-99.
- [Google Scholar]
- Pharmacogenetics of nucleoside reverse-transcriptase inhibitor-associated peripheral neuropathy. Pharmarogenomics. 2009;10:623-37.
- [Google Scholar]
- [No authors listed]. 144-week data released on Gilead's study 934 Comparing Truvada(R) to Combivir(R) Both in Combination with Sustiva. AIDS Patient Care STDS. 2007;21:603-4.
- [Google Scholar]
- Study 934 Group. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251-60.
- [Google Scholar]
- High incidence of zidovudine induced anaemia in HIV infected patients in eastern India. Indian J Med Res. 2010;132:386-9.
- [Google Scholar]
- Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr. 2008;47:74-8.
- [Google Scholar]
- The human multidrug resistance protein 4 (MRP4, ABCC4): Functional analysis of a highly polymorphic gene. J Pharmacol Exp Ther. 2008;325:859-68.
- [Google Scholar]
- Pharmacogenetic characteristics of indinavir, zidovudine, and lamivudine therapy in HIV-infected adults: a pilot study. J Acquir Immune Defic Syndr. 2006;42:441-9.
- [Google Scholar]
- Interindividual variability in pharmacokinetics of generic nucleoside reverse transcriptase inhibitors in TB/HIV-coinfected Ghanaian patients: UGT2B7*c is associated with faster zidovudine clearance and glucuronidation. J Clin Pharmacol. 2009;49:1079-90.
- [Google Scholar]
- Stavudine: A review of its pharmacodynamic and pharmacokinetic properties and clinical potential HIV infection. Drug. 1996;51:854-64.
- [Google Scholar]
- World Health Organization (WHO). Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach: 2010 revision. Geneva: WHO; 2010.
- [Google Scholar]
- HIV 2012/2013. Medizin Fokus Verlag, Hamburg. 2012. Available from: https://hivbook.files.wordpress.com/2011/10/hivbook-2012.pdf
- [Google Scholar]
- HLA-B 17 prevalence in HIV-1 infected patients under antiretroviral treatment. Int J Hum Genet. 2011;11:59-62.
- [Google Scholar]
- Evaluation of quantitative liver function tests in HIV-positive patients under anti-retroviral therapy. Eur J Med Res. 2009;14:369-77.
- [Google Scholar]
- First-line antiretroviral therapy with nevirapine versus lopinavir-ritonavir based regimens in a resource-limited setting. AIDS. 2014;28:1143-53.
- [Google Scholar]
- Clinical experience with non-nucleoside reverse transcriptase inhibitors. AIDS. 1997;11(Suppl A):S157-64.
- [Google Scholar]
- HLA involvement in nevirapine-induced dermatological reaction in antiretroviral-treated HIV-1 patients. J Pharmacol Pharmacother. 2011;2:114-5.
- [Google Scholar]
- Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology. 2002;35:182-9.
- [Google Scholar]
- Pharmacogenetics of nevirapine-associated hepatotoxicity: an adult Clinical Trials Group collaboration. Clin Infect Dis. 2006;43:783-6.
- [Google Scholar]
- Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71-5.
- [Google Scholar]
- Analyzing sleep abnormalities in HIV-infected patients treated with Efavirenz. Clin Infect Dis. 2004;38:430-2.
- [Google Scholar]
- Metabolic complications associated with HIV protease inhibitor therapy. Drugs. 2003;63:2555-74.
- [Google Scholar]
- Sexual dysfunction associated with protease inhibitor containing high active antiretroviral treatment. AIDS. 2001;15:1019-23.
- [Google Scholar]
- The relationship between HIV infection and cardiovascular disease. Curr Cardiol Rev. 2008;4:203-18.
- [Google Scholar]
- Risk-benefit of HMG-CoA reductase inhibitors in the treatment of HIV protease inhibitor-related hyperlipidaemia. Expert Opin Drug Saf. 2002;1:5-17.
- [Google Scholar]
- Heart and HAART: Two sides of the coin for HIV-associated cardiology issues. World J Cardiol. 2010;2:53-7.
- [Google Scholar]
- Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423-31.
- [Google Scholar]
- Contribution of 20 single nucleotide polymorphisms of 13 genes to dyslipidemia associated with antiretroviral therapy. Pharmacogenet Genomics. 2007;17:755-64.
- [Google Scholar]
- Change to atazanavir/ritonavir treatment improves lipids but not endothelial function in patients on stable antiretroviral therapy. AIDS. 2010;24:885-90.
- [Google Scholar]
- Safety and efficacy of a 36-week induction regimen of abacavir/lamivudine and ritonavir-boosted atazanavir in HIV-infected patients. HIV Clin Trials. 2010;11:69-79.
- [Google Scholar]
- Efficacy and safety of replacing lopinavir with atazanavir in HIV-infected patients with undetectable plasma viraemia: final results of the SLOAT trial. J Antimicrob Chemother. 2008;61:200-5.
- [Google Scholar]
- Gilbert syndrome and the development of antiretroviral therapy-associated hyperbilirubinemia. J Infect Dis. 2005;192:1381-6.
- [Google Scholar]
- Incidence of atazanavir-associated hyperbilirubinemia in Korean HIV patients: 30 months follow-up results in a population with low UDP-glucuronosyltransferase1A1*8 allele frequency. J Korean Med Sci. 2010;25:1427-30.
- [Google Scholar]
- Gilbert syndrome and glucose-6-phosphate dehydrogenase deficiency: a dose-dependent genetic interaction crucial to neonatal hyperbilirubinemia. Proc Natl Acad Sci USA. 1997;94:12128-32.
- [Google Scholar]
- Genetic factors influencing atazanavir plasma concentrations and the risk of severe hyperbilirubinemia. AIDS. 2007;21:41-6.
- [Google Scholar]
- The genetics of stone disease. In: Stoller M, Meng MI, eds. Urinary stone disease: The practical guide to medical and surgical management. Totowa, New Jersey: Humana Press; 2007. p. :35-48.
- [Google Scholar]
- Haematuria and urolithiasis in patients with haemophilia. Eur J Haematol. 2003;70:410-2.
- [Google Scholar]
- HIV integrase as a target for antiviral chemotherapy. Rev Med Virol. 2002;12:179-93.
- [Google Scholar]
- Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science. 2000;287:646-50.
- [Google Scholar]
- Potential of novel antiretrovirals to modulate expression and function of drug transporters in vitro . J Antimicrob Chemother. 2011;66:802-12.
- [Google Scholar]
- The intracellular disposition of raltegravir is dependent on P-gp (ABCB1) activity and is significantly reduced in primary CD4+ P-gp high T cells. In: Proceedings of the 19th Conference on Retroviruses and Opportunistic Infections. 2012. p. :590.
- [Google Scholar]
- Pharmacokinetics of raltegravir in individuals with UGT1A1 polymorphisms. Clin Pharmacol Ther. 2009;85:623-7.
- [Google Scholar]
- Pharmacokinetics and pharmacogenomics of once-daily raltegravir and atazanavir in healthy volunteers. Antimicrob Agents Chemother. 2010;54:4619-25.
- [Google Scholar]
- Enfuvirtide, a new fusion inhibitor for therapy of human immunodeficiency virus infection. Pharmacotherapy. 2004;24:198-211.
- [Google Scholar]
- Allosteric model of maraviroc binding to CC chemokine receptor 5 (CCR5) J Biol Chem. 2011;286:33409-21.
- [Google Scholar]
- Absence of the HIV-1 protective Delta ccr5 allele in most ethnic populations of India. Eur J Hum Genet. 2001;9:794-6.
- [Google Scholar]
- CCR5 delta32 deletion and response to highly active antiretroviral therapy in HIV-1-infected patients. AIDS. 2011;14:2788-90.
- [Google Scholar]
- CCR5Delta32 and promoter polymorphisms are not correlated with initial virological or immunological treatment response. AIDS. 2001;15:2259-66.
- [Google Scholar]
- Effects of CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers. Br J Clin Pharmacol. 2008;65(Suppl 1):27-37.
- [Google Scholar]
- Maraviroc is a substrate for OATP1B1 in vitro and maraviroc plasma concentrations are influenced by SLCO1B1 521 T>C polymorphism. Pharmacogenet Genomics. 2010;20:759-65.
- [Google Scholar]
- European guidelines on the clinical management of HIV-1 tropism testing. Lancet Infect Dis. 2011;11:394-407.
- [Google Scholar]






