Translate this page into:
Isolation & molecular characterization of human parainfluenza virus in Chennai, India
Reprint requests: Dr K. Kaveri, Department of Virology, King Institute of Preventive Medicine & Research Guindy, Chennai 600 032, Tamil Nadu, India e-mail: kaveri_raj1967@yahoo.com
-
Received: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Human parainfluenza virus (HPIV) accounts for a significant proportion of lower respiratory tract infections in children as well as adults. This study was done to detect the presence of different subtypes of HPIV from patients having influenza like illness (ILI).
Methods:
Throat and nasal swabs from 232 patients with ILI who were negative for influenza viruses were tested by multiplex reverse transcription polymerase chain reaction(mRT-PCR) for the detection of human parainfluenza virus. All samples were inoculated in rhesus monkey kidney (LLC-MK2) cell line.
Results:
Of the 232 samples, 26(11.2%) were positive by mRT-PCR and nine (34.6%) showed cytopathic effect with syncytium formation for HPIV and all were HPIV-3 serotype, other serotypes like 1,2,4 were negative. The HPIV-3 strains (HN gene) were sequenced and analysed. Two novel mutations were identified at amino acid residues 295 and 297.
Interpretation & conclusions:
The mRT-PCR assay offers a rapid, sensitive and accurate diagnostic method for detection of HPIV which enables early detection and control. In our study there was a predominance of HPIV among 1-5 yr age group and the school going age group was less affected. Further studies need to be done to characterize HPIV isolated from different parts of the country.
Keywords
Human parainfluenza virus
mRT-PCR
isolation
ILI
prevalence
Human parainfluenza virus (HPIV) is known to cause acute respiratory infections (ARI) including lower respiratory tract infection, which is a leading cause of morbidity and mortality in infants and young children world-wide123. HPIV belongs to the Paramyxoviridae family, subfamily Paramyxovirinae, and is classified into four serotypes (HPIV-1, HPIV-2, HPIV-3, and HPIV-4). Serotype 4 can be further subdivided into two antigenic subtypes, HPIV-4A and HPIV-4B4. Infection with HPIV in immuno-compromised children is known to be associated with a range of diseases, from mild upper-respiratory symptoms to severe disease requiring mechanical ventilation and leading to death5. Of the four recognized serotypes, HPIV-3 is most commonly associated with serious lower respiratory tract illness, followed by HPIV-1 and HPIV-2; HPIV-4 is rarely associated with serious illness6. HPIV is second only to respiratory syncytial virus (RSV) as a cause of hospitalizations for acute respiratory infection among children aged <5 yr; 2-17 per cent of such hospitalizations are due to HPIV infection6. It is important to know the mechanism resulting in genetic and antigenic diversity of HPIV for controlling the pathogen.
The use of classic diagnostic methods like viral isolation and serology is time consuming and takes several weeks till the results are available, and hence these methods are less useful for making therapeutic decisions7. Cell culture, often considered to be the gold standard, is delicate and sometimes too slow for it to be useful for diagnosis. Direct antigen detection methods are widely used for rapid diagnosis of HPIV infections8910 but results can be variable511. Multiplex reverse transcription polymerase chain reaction (mRT-PCR) assay can be a sensitive and specific tool for rapid diagnosis of HPIV infections51213. In this study, mRT-PCR was performed for the simultaneous detection of HPIV-1,2,3 and 4 in samples collected from patients with influenza like illness (ILI). The isolation positive samples were sequenced and analysed.
Material & Methods
Clinical samples: Throat and nasal swabs were collected during January 2011 to August 2012 from patients with ILI belonging to different age groups attending outpatient departments (OPD) of tertiary care government hospitals in Chennai, Tamil Nadu, India [Institute of Child Health and Hospital (52 samples), Royapettah Government Hospital (20 samples), Saidapet Government Hospital (26 samples), Rajiv Gandhi Government Hospital (39 samples), Government Peripheral Hospital (42 samples), Kilpauk Medical College (29 samples) and Stanley Medical College (24 samples)].
Sample collection and processing: The study was conducted in the department of Virology, King Institute of Preventive Medicine and Research, Chennai. A total of 232 throat and nasal swabs were collected from infants, children, adolescents and adults. These samples were collected from patients with symptoms like fever, chills/rigors, nasal discharge, cough, sore throat, breathelessness and headache. Clinical samples were collected in 3 ml of cold viral transport medium (Hank's balanced salt solution) containing 0.5 per cent gelatin and transported in cold chain to the laboratory.
Nucleic acid extraction: QIAmp viral Qiagen RNA extraction kit was used for RNA extraction from clinical samples and Invitrogen superscript III platinum one step RT-PCR system for the detection of RNA. Oligonucleotide primers against hemagglutinin neuraminidase (HN) gene were used to detect HPIV-1, 2, 314 and for HPIV-4, primers were directed against phosphoprotein gene15 (Table I).

Optimized reagents and PCR cycling condition for HPIV: For primary PCR amplification, RNA was added to PCR mixture containing buffer, water, Taq polymerase, primers to a final volume of 50 µl. Amplification was performed on ABI Thermal Cycler, USA, with cyclic conditions of 50°C for 30 min, 94°C for 15 min, 35 cycles of 94°C for 30 sec, 55°C for 30 sec, 72°C for one min and 72°C for 10 min for elongation14. PCR products were visualized in 1.5 per cent agarose gel electrophoresis and molecular weight marker of 100 bp used. Expected band sizes for HPIV-1,2,3 and 4 were 371, 507, 189 and 451 bp, respectively.
Virus and reagents: The human parainfluenza virus was propagated in rhesus monkey kidney cell line LLC-MK2 [National Institute of Virology (NIV), Pune] at 37°C with 5 per cent CO2 in humidified conditions. The cells were maintained in Eagles minimal essential medium (Sigma, USA), supplemented with 10 per cent foetal bovine serum (FBS, Hi media, India) and 0.01 per cent antibiotic-antimycotic solution penicillin, streptomycin, kanamycin and fungizone, and trypsin-EDTA.
Viral isolation: All samples were inoculated into tissue culture T 25cm2 flasks of LLC-MK2 cell line (NIV, Pune), allowed to adsorb for one hour and incubated at 37°C. Cell monolayers were observed for cytopathic effect (CPE) every 48 h. HPIV positive clinical isolates demonstrated focal rounding and destruction, occasional syncytia on initial isolation. The samples which showed CPE were confirmed by mRT- PCR.
Sequence analyzing: Three representative HN genes of HPIV were detected by mRT-PCR and isolation positive samples were sequenced and analysed. The HPIV-3 standard strain was downloaded along with strains from different countries from NCBI database (www.ncbi.nlm.nih.gov). Strain-gi/168481518/swine/USA/2009 was used as out group for construction of phylogenetic tree. All the sequences were analyzed by MEGA (version 5) program (mega.software.informer.com/5.0/) using maximum likelihood method with p distance16.
Results
Evaluation of multiplex reverse transcription PCR with clinical specimens: Oligonucleotide primers were used to amplify HN gene of HPIV (Table I). A total of 232 samples were tested for HPIV by mRT-PCR, of which 26 (11.2%) were positive for HPIV-3, other serotypes were negative. Positive samples showed amplicon size 189 bp for HPIV-3 (Fig. 1). Twenty mRT-PCR positives were among the paediatric age group (less than 12 yr) and six positives were in the group more than 12 yr.
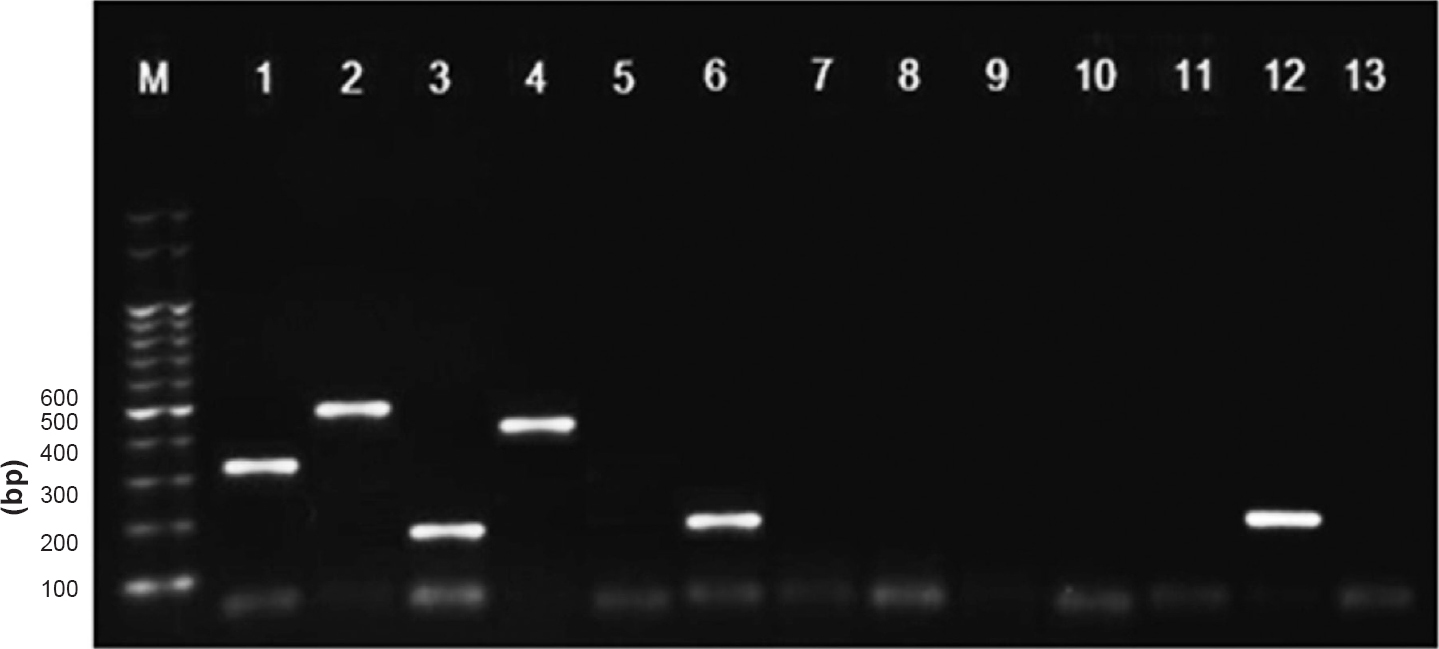
- Results of multiplex reverse-transcription (RT)-PCR with clinical samples and controls. Lane M, Marker; Lane 1, HPIV-1(Positive control); Lane 2, HPIV-2(PC); Lane 3, HPIV-3(PC); Lane 4, HPIV4(PC); Lane 5, Negative; Lane 6, HPIV3(clinical sample); Lanes 7 to 11, Negative; Lane 12, HPIV-3 (clinical sample); Lane 13, Negative control
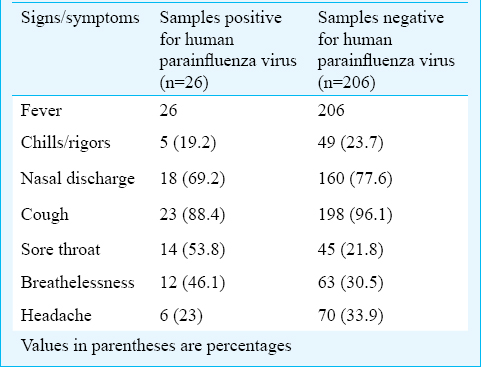
Symptoms analyses for HPIV cases: In this study, 151 males were tested and 16 (10.5%) were positive. Among the 81 females tested, 10 (12.3%) were positive for HPIV-3 by mRT-PCR. Among all the signs and symptoms presented by the HPIV positive cases sore throat and, breathlessness were significant when compared with the negative cases (P<0.05) (Table II).
Comparison of multiplex reverse transcription PCR with tissue culture techniques: Viral isolation was attempted using LLC-MK2 cell lines. Of the 232 samples subjected to viral isolation in LLC-MK2, nine (34%) were positive. Negatives were discarded after three passages, and the samples that showed mild changes in the cell morphology were passaged further. If these samples produced clear CPE these were confirmed by PCR, if CPE was not seen after two more passages, these were checked by PCR and if negative, discarded. Clear CPE was observed in the ninth passage in seven samples and two samples showed CPE in 5th passage. These samples were reconfirmed by RT-PCR as HPIV-3.
Virus and sequence analysis: Among the 26 positives, three representative samples from different age groups and three geographical regions within Tamil Nadu were chosen and subjected to sequencing. The sequences of HPIV-3 strains were submitted to NCBI. Their accession numbers are (JQ901411-JQ901413).
Comparison of sequence with other strains
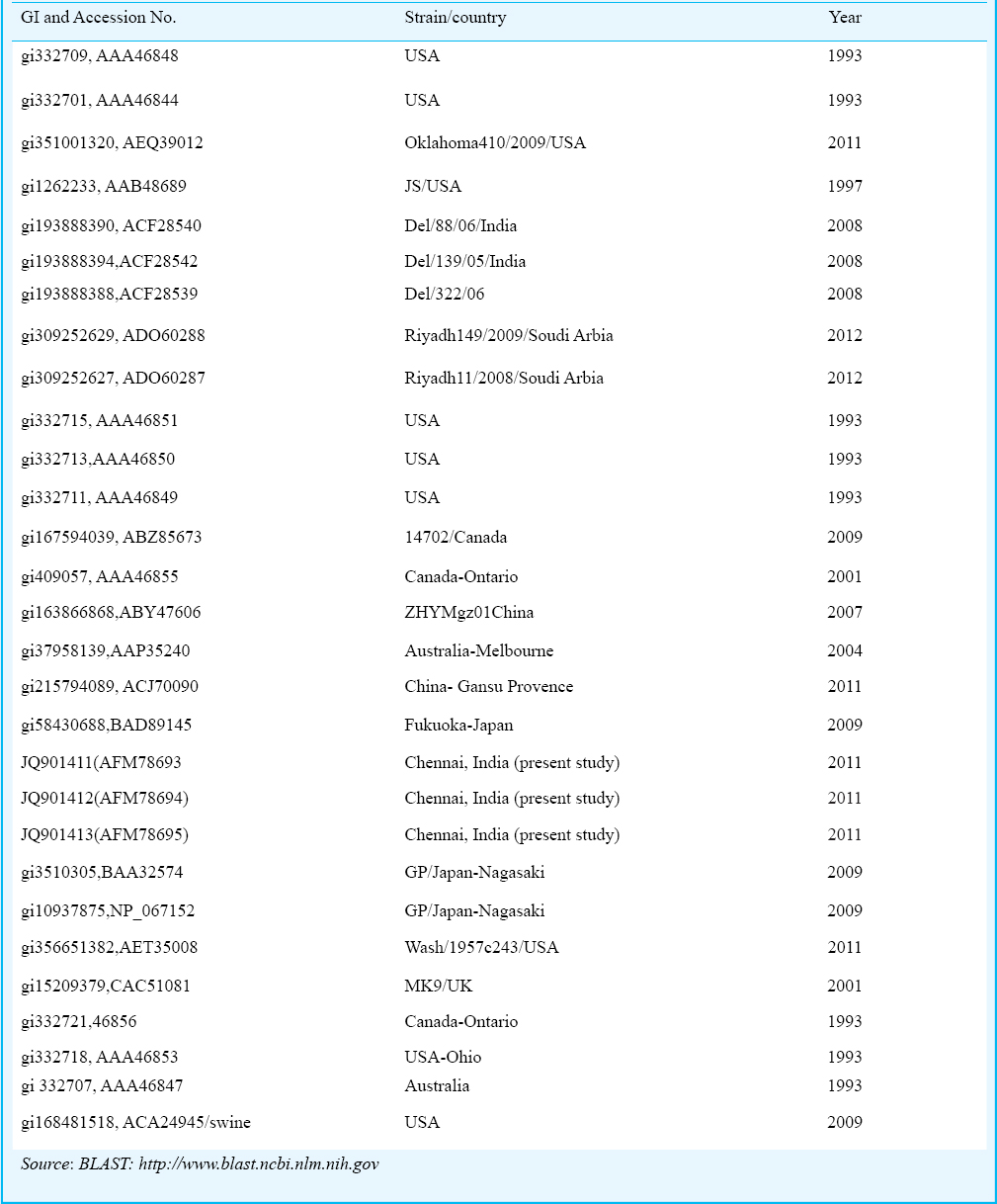
Sequences of HPIV strains from different countries were retrieved and compared with our strains using BLAST (blast.ncbi.nlm.nih.gov). (Table III). Phylogenetic analysis of our strains (Fig. 2) showed that these were highly similar to strains from Nagasaki, Fukuoka and Melbourne strains (97% similarity) and formed a same clade, The other strains that were significantly related were Washington (1973 and 1979), Oklahoma (2009), Bethesda (1997), Delhi (2005 and 2006), Riyadh (2008 and 2009), Texas (1980, 1982 and 1983), Logan (2009), Ontario (2001) and Guangdong (2007). The similarity confirmed the circulation of HPIV in Chennai and also its similarity to the strains in different countries.
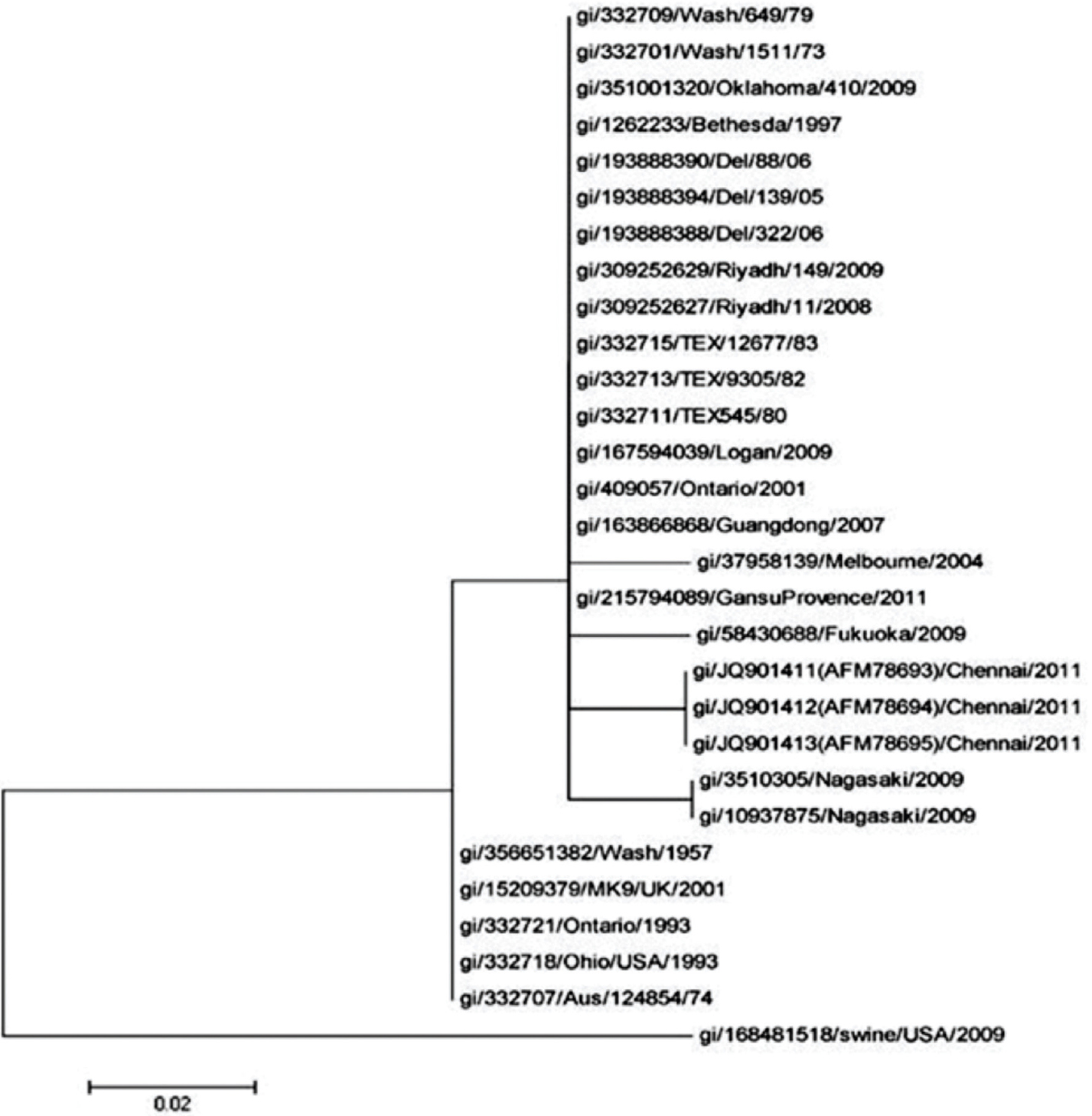
- Phylogenetic analysis of the deduced amino acid sequences of hemagglutinin neuraminidase gene of HPIV-3 to members of the family Paramyxoviridae. The tree was constructed by the maximum likelihood method with p distance.
Amino acid analysis: In our strains two mutations were identified at 295 and 297 amino acid residue. At 295 residue, glycine was replaced by serine, which can act as protein functional centres and hydrophobic amino acid. At position 297, histidine was replaced by tyrosine. Histidine is an essential amino acid with a positively charged imidazole functional group.
Discussion
It is known that among respiratory viruses namely RSV, HPIV and influenza viruses A and B, RSV has been documented to be the most common pathogen17 followed by human influenza virus and HPIV. Among the HPIVs, serotype 3 has been predominantly reported218 as also found in the present study.
In our study HPIV positivity was seen during the monsoon months of August-September and post monsoon months of November-January, with peak positivity in November. But in northern hemisphere HPIV infections were more frequent from January to April19. In temperate climates, HPIV-3,4 were detected in spring, summer and late fall and seasonal incidence varied for HPIV-1,2 in Chinese children20.
A high proportion of males were found to be infected with respiratory viruses as compared to females in our study which was similar to another study in Delhi21. The patients’ median age was 20 months for HPIV-4 infections and 7-11 months for HPIV-1, 2 and 3 infections, but the clinical manifestations did not differ significantly between HPIV-1, -2, -3, and -4 infections20. It is known that influenza virus infections are more common in the paediatric age group, likewise in our study there was a predominance of HPIV among 1-5 age group. HPIV-3 was the prevalent serotype.
In this study the most common mode of presentation was fever, followed by sore throat and cough like any other ILI. Since samples were not collected from patients with SARI (severe acute respiratory illness) and long term follow up was not done, the rate of hospitalization among the ILI cases was not assessed in our study. Additional studies that include hospitalized controls are needed to clarify the clinical importance of HPIV infection in adults with community-acquired lower respiratory tract infection. One of the limitations of our study was that screening for RSV was not undertaken, as this could have been of great relevance in terms of the role of other respiratory pathogens in causing ILI.
In our study mRT-PCR assay was able to identify a greater number of positives in clinical specimens than cell culture as reported earlier41115. Of the 26 specimens that were mRT-PCR positive, only nine HPIV-3, strains could be isolated which was about one third of the total. Detection of HPIV in the remaining 17 specimens by mRT-PCR and not by isolation was due to the high sensitivity of mRT-PCR assay22. Hence compared to other methods, mRT-PCR seemed to be a better method for detecting HPIV in suspected cases. There are similar studies indicating mRT-PCR to be a better diagnostic aid when compared with viral isolation and immunoflouresence tests15. The advantages are that mRT-PCR does not require cell line maintenance, as the isolation of HPIV requires multiple passages which is not only time consuming but also cost ineffective.
It was earlier identified that the point mutation at residues 278 and 281 coded for a single amino acid substitution in the HN protein23. Another study also conferred the mutations at the residues in threonine 193 isoleucine and isoleucine 567 valine24. The viral neuraminidase alters the host cells surface, modulating the number of available sialic acid receptors and thus determining the outcome of infection, fewer sialic acid receptors are available to interact with other viral HN molecules. Other studies have shown one point mutation in the HN gene corresponding to a single amino acid change in the HN glycoprotein, which converts aspartic acid 216 to an asparagine and proline 111 to a serine2526. We found two novel mutations at amino acid residues of 295 and 297.
In conclusion, mRT-PCR assay could be used for the accurate diagnosis and detection of HPIV. Further, expanded surveillance throughout the country will help in better epidemiological analysis, for implementation of better public health programmes in controlling virus induced respiratory infections.
Acknowledgment
Authors acknowledge the Director, Institute of Child Health and Hospital for Children and Deans, Royapettah Government Hospital, Rajiv Gandhi Government Hospital, Kilpauk and Stanley Medical College for permitting to collect samples from patients, and thank the Superintendent of Saidapet Government Hospital and Government peripheral Hospital for cooperation.
References
- Identification of natural human serotype 3 parainfluenza virus. Virol J. 2011;8:58.
- [Google Scholar]
- Epidemiological features of parainfluenza virus infections: laboratory surveillance in England and Wales, 1975-1997. Eur J Epidemiol. 1999;15:475-84.
- [Google Scholar]
- Prevalence of human respiratory viruses in adults with acute respiratory tract infections in Beijing, 2005-2007. Clin Microbiol Infec. 2009;15:1146-53.
- [Google Scholar]
- Human parainfluenza virus 4 outbreak and the role of diagnostic tests. J Clin Microbiol. 2005;43:4515-21.
- [Google Scholar]
- Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. J Clin Invest. 2005;115:1688-98.
- [Google Scholar]
- Seasonal trends of human parainfluenza viral infections: united states, 1990-2004. J Clin Infect Dis. 2006;43:1016-22.
- [Google Scholar]
- Simultaneous detection and identification of human parainfluenza viruses 1, 2 and 3 from clinical samples by multiplex PCR. J Clin Microbiol. 1998;36:1388-91.
- [Google Scholar]
- Comparison of monoclonal time-resolved fluoroimmunoassay with monoclonal capture-biotinylated detector enzyme immunoassay for adenovirus antigen detection. J Clin Microbiol. 1989;25:1662-7.
- [Google Scholar]
- Type specific detection of parainfluenza viruses by enzyme-immunoassay and radioimmunoassay in nasopharyngeal specimens of patients with acute respiratory disease. J Gen Virol. 1981;56:49-57.
- [Google Scholar]
- Detection of respiratory viruses in nasopharyngeal secretions with immunofluorescence technique for multiplex screening an evaluation of the Chemicon assay. Clin Diagnostic Virol. 1996;6:147-54.
- [Google Scholar]
- Identification of adenovirus, influenza virus, prainfluenza virus, respiratory syncytial virus by two kinds of Multiplex polymerase chain reaction (PCR) and a shell vial culture in pediatric patients with viral pneumonia. Yonsei Med J. 2010;51:761-7.
- [Google Scholar]
- Rapid detection of parainfluenza virus type 3 RNA in respiratory specimens: use of reverse transcription-PCR-enzyme immunoassay. J Clin Microbiol. 1994;32:484-8.
- [Google Scholar]
- Molecular epidemiology of a parainfluenza type 3 virus outbreak on a pediatric ward. J Infect Dis. 1993;167:1441-5.
- [Google Scholar]
- Development of the multiplex RT-PCR assays for the detection of 12 respiratory RNA viruses. J Virol Methods. 2005;126:53-63.
- [Google Scholar]
- Detection and identification of human parainfluenza viruses 1, 2, 3 and 4 in clinical samples of pediatric patients by multiplex reverse transcription-PCR. J Clin Microbiol. 2000;38:1191-5.
- [Google Scholar]
- MEGA5 Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum distance and maximum parsimony methods. Mol Biol Evol. 2011;28:2731-9.
- [Google Scholar]
- Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917-28.
- [Google Scholar]
- A prospective three-year cohort study of the epidemiology and virology of acute respiratory infections of children in rural India. PLoS One. 2007;2:e491.
- [Google Scholar]
- Rapid detection of respiratory viruses by centrifugation enhanced cultures from children with acute lower respiratory tract infections. J Clin Virol. 2000;16:41-7.
- [Google Scholar]
- Human parainfluenza virus type 4 infection in Chinese children with lower respiratory tract infections: A comparison study. J Clin Virol. 2011;51:209-12.
- [Google Scholar]
- Respiratory viral infections detected by multiplex PCR among pediatric patients with lower respiratory tract infections seen at an urban hospital in Delhi from 2005 to 2007. Virol J. 2009;6:89.
- [Google Scholar]
- Superiority of reverse-transcription polymerase chain reaction to conventional viral culture in the diagnosis of acute respiratory tract infections in children. J Infect Dis. 2004;189:706-10.
- [Google Scholar]
- Antigenic and structural properties of the HN glycoprotein of human parainfluenza virus type 3 sequence analysis of variant selected with monoclonal antibodies which inhibit infectivity, hemagglutination and neuraminidase activity. J Virol. 1987;61:1473-7.
- [Google Scholar]
- A single amino acid alterations in the human parainfluenza virus-3 hemagglutinin neuraminidase glycoprotein confers resistance to the inhibitory effects of Zanamivir on receptor binding and neuraminidase activity. J Virol. 2001;75:6310-20.
- [Google Scholar]
- Hemagglutinin neuraminidase of human parainfluenza virus 3; role of the neuraminidase in the viral life cycle. J Virol. 1995;214:294-300.
- [Google Scholar]
- Human parainfluenza virus type 3 HN-receptor interaction:effect of 4-Guanidino-Neu5Ac2en on a neuraminidase deficient variant. J Virol. 2001;75:7481-8.
- [Google Scholar]






