Translate this page into:
Status of hepatitis B infection - a decade after hepatitis B vaccination of susceptible Nicobarese, an indigenous tribe of Andaman & Nicobar (A&N) islands with high hepatitis B endemicity
Reprint requests: Dr A.P. Sugunan, Regional Medical Research Centre (ICMR), Department of Health Research, Ministry of Health & Family Welfare, Post Bag No.13, Port Blair 744 101, Andaman & Nicobar Islands, India e-mail: sugunanap@icmr.org.in; apsugunan@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Andaman and Nicobar Islands of India, home to six primitive tribes, constituting about 10 per cent of the total population of these Islands have been detected with high endemicity of hepatitis B infection. During 2000, a total of 936 individuals ≤ 45 yr, negative for hepatitis B surface antigen (HBsAg) and antibody anti-HBs were vaccinated with three doses of a recombinant DNA hepatitis B vaccine in two villages of Car Nicobar Islands. The present study was undertaken to evaluate the impact of the hepatitis B vaccination with respect to the persistence of antibodies and incidence of new infections, prevalence of surface gene mutations among the Nicobarese community in the two villages ten years after hepatitis B vaccination.
Methods:
Follow up samples were collected from 211 individuals who had received three doses of vaccine ten years back and from a control group of 515 non-vaccinated individuals. The HBsAg, anti-HBs and anti-HBc assay results were compared among vaccinated and non-vaccinated groups. HBV DNA was extracted and sequenced from all the samples for detection of mutation. Genotyping and serotyping of the viruses were performed.
Results:
The results showed that 85.3 per cent of the vaccinated persons retained protective level of antibodies and among the non-vaccinated individuals, 54.2 per cent showed presence of anti-HBs indicating an exposure to the infection. The overall HBsAg positivity among the studies Nicobarese individuals was reduced to 7.4 per cent after 10 years of vaccination. Anti-HBc was positive in 60.6 and 57 per cent among the vaccinated and non-vaccinated individuals, respectively. Overall breakthrough infection of 8.5 per cent was detected among the vaccinated individuals. The predominant genotype and serotype circulating among these tribal populations were D and ayw3, respectively.
Interpretation & conclusions:
The results of this study showed an overall reduction in the pool of HBsAg carriers because of the vaccination which helped in reducing the HBsAg carrier rate among the non-vaccinated also, probably due to an increase in herd immunity and reduction in the source of infection. Further studies need to be done to evaluate long term benefits of hepatitis B vaccination among these tribes.
Keywords
Breakthrough infection
genotype
HbsAg
HBV
Nicobarese
seroprotected
vaccine
Hepatitis B virus (HBV) infection is a global health problem. The disease is an important public health problem in India also with an estimated national carrier rate of 4.7 per cent1. The Global Advisory Group of the Expanded Programme on Immunization recommended inclusion of the hepatitis B vaccine in the National Immunization Programme in 1989 which was endorsed by the World Health Organization (WHO) in 1992. Hepatitis B vaccine (both plasma derived and recombinant) has been shown to be highly efficacious in preventing infection with hepatitis B virus3.
The six primitive tribes of Andaman and Nicobar (A&N) Islands, India, constitute about 10 per cent of the total population of these Islands. Hepatitis B infection is highly endemic among the tribes of these Islands with HBsAg (hepatitis B surface antigen) rates of 23.3 per cent among the Nicobarese (a mongoloid tribe that constitutes more than 98% of the tribal population), which is perhaps the highest reported rate in India4. In 2000-2001, a pilot project of mass hepatitis B vaccination using an indigenously developed recombinant DNA vaccine was initiated in two villages of Car Nicobar Islands inhabited exclusively by Nicobarese5. The total population of Car Nicobar Island is around 17,125 (Census 2011)5 which is distributed in 15 villages. The vaccination programme was carried out in two villages of Car Nicobar having a total population of 2376. A total of 936 individuals aged 45 yr or less and negative for HBsAg and anti-HBs were vaccinated with three doses of Shanvac-B5. The vaccine was well tolerated without significant side effects. None of the subjects had any anaphylactic reaction. More than 95 per cent of the vaccinated people developed anti-HBs titre of ≥10mIU/ml, indicating seroprotection after the third dose. The proportion of people with seroprotection dropped to about 85.5 per cent in three years after the vaccination6.
The objective of the present study was to evaluate the level of seroprotection and incidence of new infections, prevalence of surface gene mutations among the Nicobarese population in the two villages 10 years after the vaccination.
Material & Methods
The study was approved by the Institutional Ethics Committee of the Regional Medical Research Centre at Port Blair, A&N Islands. The Tribal Council and the village chieftains of Car Nicobar Islands were informed about the study and their consent was obtained. The study was carried out during August 2010 to August 2012 as field surveys conducted in the two villages of Car Nicobar Island where the vaccinated people have been living.
Samples size and sampling procedure: A prevalence of 15 per cent for antibody levels below protective level (10 mIU/ml) was assumed based on earlier surveys67. To estimate this prevalence with an absolute precision of 5 per cent with 95% confidence limits, a sample size of 196 was required. A 10 per cent oversampling was done and a sample size of 220 vaccinated individuals was targeted.
A 5 per cent prevalence of infection with mutant strains of HBV among the non-vaccinated persons was assumed based on experience in neighbouring Southeast Asian countries8. To estimate such prevalence with 2 per cent absolute precision a sample size of 456 non-vaccinated individuals was required. The sample size was inflated allowing for an assumed 10 per cent non-participation by selected subjects. The non-vaccinated individuals were selected from among the population of the two villages randomly. Both the groups were not matched for gender and age.
Patients & samples: Blood samples (5 ml) were collected from vaccinated and non-vaccinated individuals. They were interviewed using a structured questionnaire, which sought information about medical conditions, risk factors and vaccination status. Serum was separated from the blood samples and stored at -80°C until processed.
Detection of serological markers: The serum samples were tested for HBsAg and anti-HBc (hepatitis B core antibody) using qualitative ELISA (General Biologicals, Taiwan) following the procedures described by the manufacturer. Anti-HBs antibody levels were estimated using a quantitative ELISA (General Biologicals, Taiwan).
PCR amplification of the HBV DNA S gene: HBV DNA was extracted from 200 µl serum samples by proteinase K/phenol : chloroform : isoamylalcohol precipitation method9. The isolated DNA was stored at - 20°C until use. Part of S gene of the extracted HBV DNA was amplified by nested PCR10 using two sets of primers. PCR was performed on 5 µl of DNA extract in a 50 µl reaction mix containing a final concentration of 10 mM Tris-HCl (Tris with 15 mM MgCl2, pH 8.3), 0.25 µM each of the four dNTPs, 2.5 U Taq DNA polymerase (Banglore Genei, India) and 0.6 mM each of the primers. DNA amplification was carried out in a GeneAmp PCR system 2720 (Applied Biosystem, USA) by using the following conditions for the first-round PCR: 5 min at 94°C, followed by 30 cycles of 94°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 1 min. Primers (Sigma, India) used for first-round PCR were 5’-ACCCCTGCTCGTGTTACAGGC-3’ (sense, nt 184-204) and 5’-AAAGCCAGACAGTGGGGGAAA-3’ (antisense, nt 731-711). For second-round PCR, 1 ml of the first-round PCR product with primers 5’-GACTCGTGGTGGACTTCTCTC-3’ (sense, nt 251–271) and 5’-TAAACTGAGCCAGGAGAAACG-3’ (antisense, nt 679-659) (concentration of primers and PCR reactants was identical to those used for first-round PCR) was subjected to 25 cycles of amplification using the first-round PCR protocol to obtain a 429 bp product.
DNA extracted from the serum samples of healthy controls and sterile water were used as the negative control. Strict precautions were followed to avoid cross-contamination and appropriate negative and positive controls were included during DNA extraction and PCR amplification steps.
Analysis of sequence data and phylogenetic analysis: The forward and reverse sequences acquired from sequencer were checked and manually edited in the electro-pherograms using the SeqScape v2.5 (Applied Biosystem, USA) and MEGA511. Genetic distances were calculated using the Kimura two parameter algorithm and phylogenetic trees were constructed by the neighbor joining (NJ) method11. To confirm the reliability of the pair-wise comparison and phylogenetic tree analysis, bootstrap resampling and reconstruction were carried out 1000 times. Phylogenetic analysis was done using MEGA511.
HBV genotyping and serotyping: Genotyping was performed on the basis of phylogenetic relationship taking 363 nt fragment sequences along with representative reference sequences from different genotypes as described earlier1213. Sequences were edited, aligned and analysed using MEGA511.
Subtyping of the HBV isolates was carried out on the basis of presence of amino acid residues at codon 122, 127 and 160 of the S gene region.
A non responder was defined as an individual having anti HBs titres of ≤10 mIU/ml. A hyporesponder showed detectable titres of anti-HBs in the range of 10-99.9 mIU/ml, and individuals having detectable antibodies ≥100 mIU/ml were considered hyper-responders. Breakthrough infection was defined as either HBsAg or HBV DNA positivity in vaccinated individuals.
Statistical analysis: The data obtained were statistically analysed by chi-square test using Epi Info 7 software (www.cdc.gov/epiinfo/).
Results
Although a sample of 220 vaccinated persons was required, but only 211 could be recruited, which is about 96 per cent of the set sample size. This included 69 males and 142 females who had received three doses of vaccine ten years back. The control group included a total of 515 non-vaccinated (244 male and 271 female) subjects.
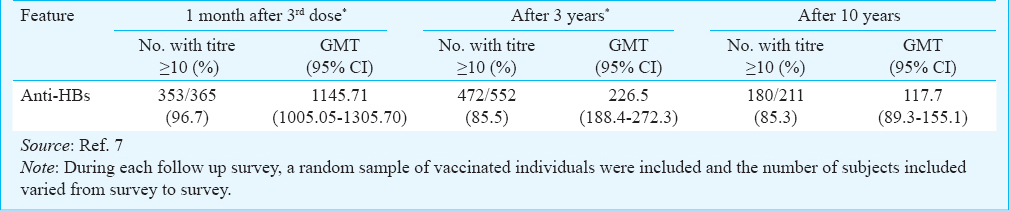
Antibody against hepatitis B surface antigen (anti-HBs): Among the 211 vaccinated persons tested for anti-HBs antibodies, 180 (85.3%, 95% CI: 80.9, 90.6) had protective levels of antibodies (≥10 mIU/ml) (Table I), which included 49 (27.2%) hyporesponders and 131 (72.8%) hyper-responders. A total of 31 (14.7%) individuals were non-responders. Among the vaccinated male subjects, 86.6 per cent (60/69; 95% CI: 73.0, 89.6) had seroprotection while among vaccinated females 84.7 per cent (120/142; 95% CI: 78.3, 89.7) had seroprotection. This difference was not significant. The age-wise presentation of anti-HBs titre showed highest protection in the age group of 50-59 yr (91.2%) and lowest among the individuals belonging to the age group of above 60 yr (64.3%) (Fig. 1). The geometric mean titre (GMT) and median titre of anti-HBs were 117.7 and 269 mIU/ml, respectively among vaccinated individuals. The highest titre (510 mIU/ml) was recorded for the age group of 20-29 yr.
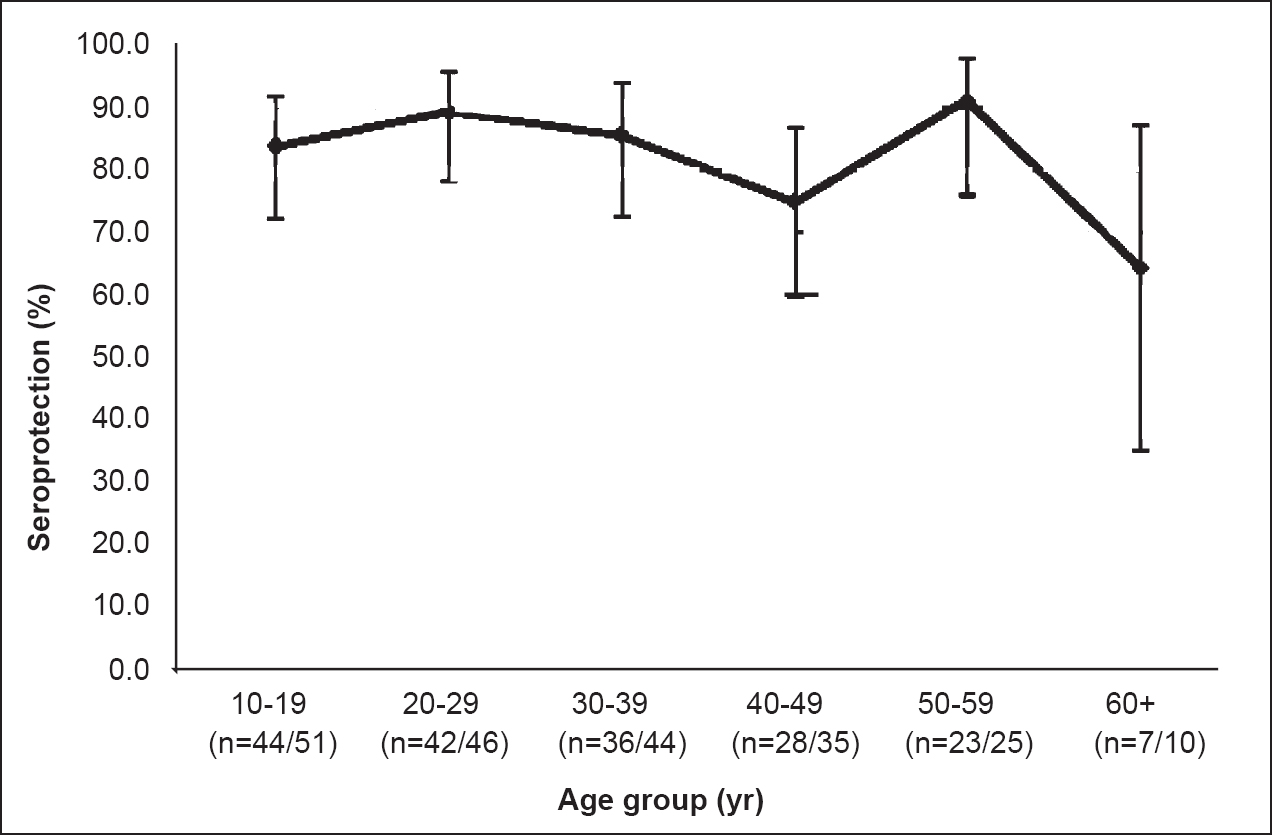
- Seroprotection level in vaccinated persons by age group.
Among the 515 non-vaccinated persons tested, 276 (53.6%, 95% CI: 49.2, 58.0) were anti-HBs positive and 239 (46.4%) were anti-HBs negative. The GMT and median titre of anti-HBs were 18.9 and 13 mIU/ml, respectively among non-vaccinated individuals. The anti-HBs positivity was higher among females (54.3%) in comparison with males (45.7%). Anti-HBs titre was found to be highest (62.1%) in the age group of 40-49 yr and lowest (46.2%) in the age group of 10-19 yr.
HBsAg positivity: Among the 726 study subjects, 54 (7.4%) were positive for HBsAg. HBsAg positivity was 2.4 per cent (5/211) among the vaccinated persons and 9.5 per cent (49/515) among the non-vaccinated persons (Table II) and the difference was significant (P<0.001). HBsAg positivity was found to be higher among males than in females in all age groups of both vaccinated and non-vaccinated groups. While the HBsAg positivity was highest (9%, 4/44) in the age group of 30-39 yr among the vaccinated persons, among the non-vaccinated persons it was highest (17.3%, 17/98) in the age group of 20-29 yr. The age group of 0-19 yr showed lowest carrier rate of HBsAg among both vaccinated and non-vaccinated groups. All the vaccinated individuals positive for HBsAg were non-responders, whereas 1.4 per cent (4/276) non-vaccinated people were positive for both HBsAg and anti-HBs.
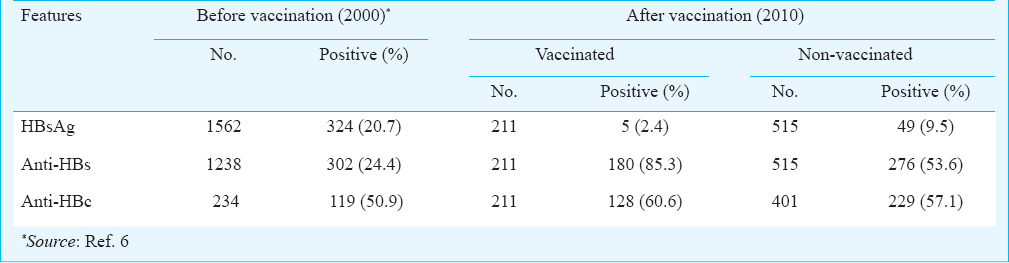
Anti-HBc positivity: All the 211 vaccinated individuals were tested for total anti-HBc antibody and 128 (60.6%) were found to be positive (Table II). Eighty two per cent (105/128) of anti-HBc positive individuals were seroprotected and 18 per cent (23/128) were non-responders. Anti-HBc positivity was 59.2 per cent (84/142) in females and 63.7 per cent (44/69) in males. A subgroup of 401 non-vaccinated subjects were tested for total anti-HBc antibody, of whom 229 (57%), were found to be positive (Table II). The anti-HBc positivity was found to be equal among females (57%) and males (57%) in this group. A total of 10.1 per cent (6.6% among vaccinated and 11.9% among non-vaccinated) individuals were positive for anti-HBc alone (negative for HBsAg, anti-HBs and HBV DNA).
Presence of HBV DNA: PCR was done in all 726 individuals. Among them, 98 (13.5%, 95% CI: 11.1, 16.2) were positive for HBV DNA, of whom 16 (16.3%; 16/98) were from vaccinated and 82 (83.7%; 82/98) from non-vaccinated individuals. Among the 98 PCR positive persons, only 36 (36.7%) were HBsAg positive.
Among the 211 vaccinated individuals, 180 had protective levels of antibody and among them 10 (5.6%) also were positive for HBV DNA. Whereas among the remaining 31 vaccinated persons who did not have protective levels of antibody, six (19.3%) were positive for HBV DNA.
Among the 515 non-vaccinated persons, 276 had protective levels of antibody and among them 24 (8.7%) were positive for HBV DNA. Whereas, among the remaining 239 non-vaccinated persons who did not have protective levels of antibody, 58 (24.3%) were positive for HBV DNA.
Breakthrough infection: Breakthrough infection (positive for S-gene PCR or ELISA for HBsAg or both) was detected among 18 (8.5%) vaccinated Nicobarese individuals (Table III). Of these 18 individuals, 13 (72%) were HBsAg negative but HBV DNA positive, three (17%) were positive in both HBsAg ELISA and for HBV DNA by PCR and two (11%) were HBV DNA negative but positive for HBsAg. All five HBsAg positive persons were vaccine non-responders. Among the 13 HBV DNA positive but HBsAg negative persons, three were vaccine non-responders and among the remaining 10, nine were hyperresponders and one hyporesponder. The HBsAg carrier rate among vaccine non-responders was 16.1 per cent (5/31, 95% CI: 6.1, 34.5) while that among vaccine responders was 0 per cent.
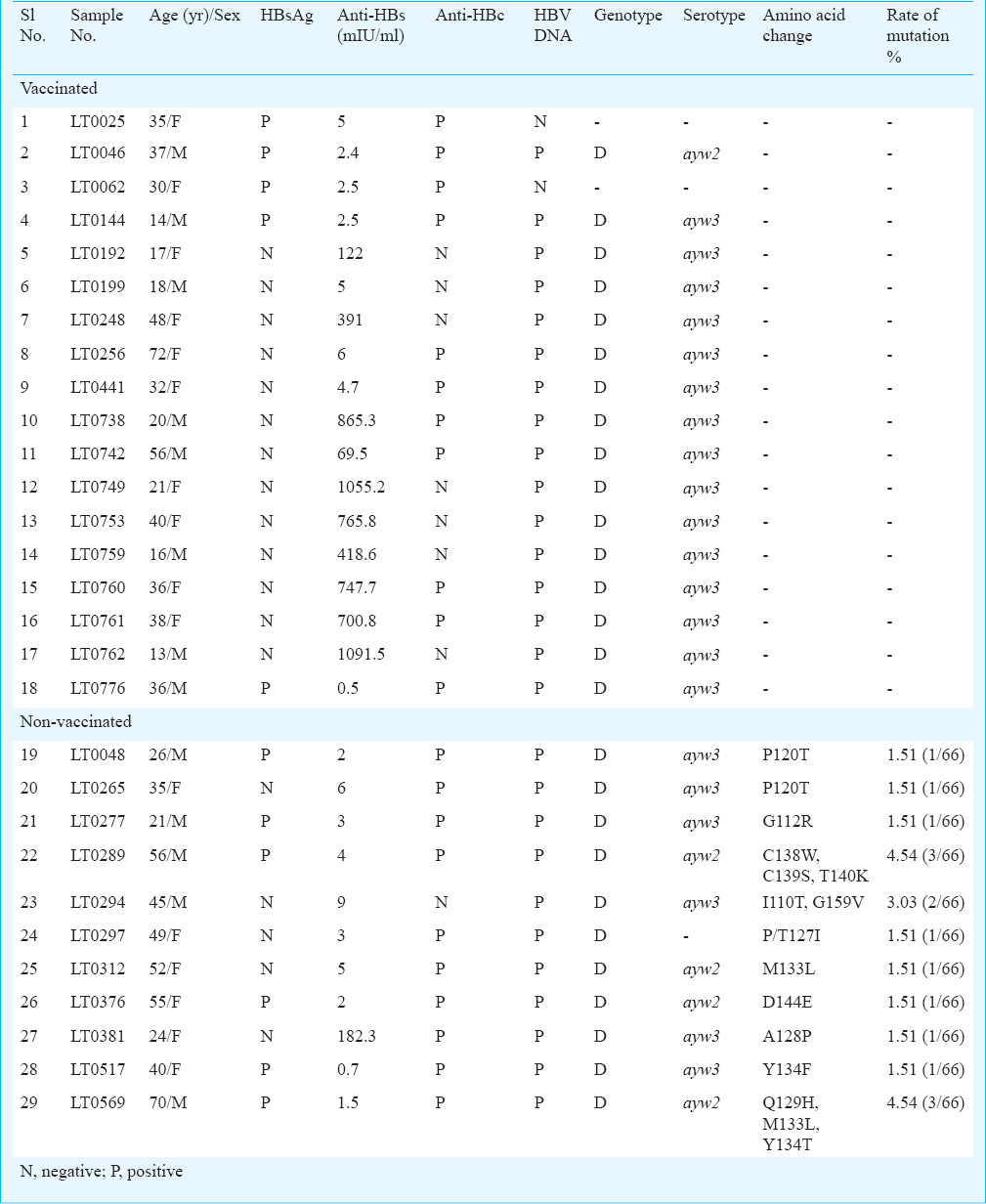
Genotyping and serotyping of hepatitis B virus: Bidirectional sequencing of partial S gene was successfully achieved for 82 (out of 98) samples, which included 16 from vaccinated and 66 from non-vaccinated individuals. Among the vaccinated individuals, all the HBV strains belonged to genotype D (Fig. 2) and serotype ayw3. Among the 66 HBV strains from non-vaccinated, 65 (98.5%) belonged to D genotype and one to genotype A (Fig. 2). Of the 65 HBV strains with D genotype, ayw3 was the major (43/65, 66%) serotype and 21 (32%) strains belonged to ayw2 serotype, and one with A genotype belonged to serotype adw2. Serotype could not be detected for one isolate due to an amino acid change at the serotype determining position.

- Phylogenetic analysis of the partial S gene showing the genotype of the 82 HBV virus from Nicobarese tribe (LT) (V denotes from vaccinated cases).
Mutation in surface region of HBV: No mutation was observed in the S gene of the vaccinated individuals. Among the 66 HBV positive samples from non-vaccinated subjects, 11 (16.7%) were detected with mutation in S gene. A total of 14 different amino acid changes were detected in the S gene of the hepatitis B virus belonging to D genotype (Table III). The rate of surface gene mutation among the non-vaccinated individuals was 24.24 per cent. Among these 11 subjects, only one had elevated level of anti-HBs titre (182.3 mIU/ml). The S gene of the HBV virus from this sample had A128P mutation (Table III). Two samples were detected with mutation leading to amino acid substitution P120T. Two samples had amino acid substitution M133L. One sample had amino acid substitution P/T127I at serotype determination position. Two samples were detected with three mutations and one with double mutations each in the S gene (Table III). Eight isolates had single mutation each viz., G112R, D144E, Y134T, A128P, M133L, P120T and P/T127I.
Discussion
Among the Nicobarese tribe the seroprotection rate observed was 96.7 per cent after 3rd dose of vaccine and 85.5 per cent after three years of vaccination, respectively67. In a follow up conducted five years after vaccination in a subsample of the same vaccinated persons, the seroprotection observed was 85.9 per cent14. The results of the present study showed that 85.3 per cent of the vaccinated persons still retained protective level of antibodies, indicating no substantial reduction in seroprotection among the vaccinated persons from third year after vaccination till ten years. However, the geometric mean antibody titre has been steadily declining during this period. This further shows that while the antibody titres decline rapidly during the initial years after vaccination, the rate of this decline decreases greatly as the antibody levels approach the minimum protective level. Among the non-vaccinated individuals, 54.2 per cent showed presence of anti-HBs indicating an exposure to the infection. Although the GMT of anti-HBsAg (117.6 mIU/ml) of vaccinated individuals in the present study was lower than that observed after 2nd and 3rd years (347.2 mIU/ml and 226.5 mIU/ml, respectively) of vaccination, no further reduction was observed in the anti-HBs titres thereafter7. The significant decrease in seroprotection rates and geometric mean titres with increase of age, possibly reflects waning of anti-HBs titre over time1516. Protection of 87 per cent was demonstrated among the participants of a study in Alaska in a 22 year follow up study3. The seroprotection rate of the HBV vaccine in South Africa was 86.8 per cent five years post vaccination16.
The overall HBsAg positivity among the Nicobarese tribals which was 20.7 per cent before vaccination reduced to 7.4 per cent after 10 years. The HBsAg positivity was 2.4 and 9.5 per cent among the vaccinated and non-vaccinated individuals, respectably indicating a substantial decrease in the HBsAg carrier rate among both the groups. Although the HBsAg positivity among the vaccinated individuals reported in this study was found to be slightly higher than elsewhere reported151718, the presence of infection among the susceptible individuals (9.5%) was much lower than that found in pre-vaccination period7. It appears that the overall reduction in the pool of HBsAg carriers because of the vaccination has helped in reducing the HBsAg carrier rate among the non-vaccinated also, probably due to an increase in herd immunity and reduction in the source of infection. Among non-vaccinated population, 1.4 per cent were positive for both HBsAg and anti-HBs but none of the individuals had detectable levels of HBV DNA. An explanation for the co-existence of anti-HBs and HBsAg could be the low ability of binding of anti-HBs to HBsAg17.
Our study revealed the presence of anti-HBc as 60.6 and 57 per cent among the vaccinated and non-vaccinated individuals, which was lower than the pre-vaccination positivity reported earlier4. As reported earlier7, our study showed high anti-HBc positivity among the vaccine responders. In the pre-vaccination period anti-HBc positivity was found to be high4 among the Nicobarese which revealed that besides a high proportion of HBsAg positivity, almost half of the tribal population negative for HBsAg and anti-HBs was positive for antibodies against hepatitis B core antigen19. As Anti-HBc positivity among the Nicobarese population was found to be high before vaccination4, only HBsAg and HBV DNA were taken as markers of breakthrough infection and individuals positive for anti-HBc after vaccination are likely to be infected before vaccination. After the third year of vaccination the rate of breakthrough infection in respect to HBsAg positivity was 1.8 per cent7, which increased to 2.4 per cent after 10 years. All the vaccinated individuals showing presence of HBsAg were non-responders. Co-existence of both HBsAg and anti-HBs was not observed among them whereas three among the five HBsAg non-responders showed presence of HBV DNA indicating a re-infection.
Genotypes and serotypes are useful tools in understanding the epidemiology of HBV infection. The present study demonstrated that the predominant genotype and serotype circulating among these tribal populations were D and ayw3, respectively, similar to those reported earlier among this community10. Surface mutations are clinically important in both HBV infection prevention (through vaccination) and diagnosis. In contrast to earlier report20, our study demonstrated high occurrence of surface mutants among non-vaccinated than in vaccinated individuals. The P120T detected in two patients was earlier described as an important substitution and was found to be linked with chronic hepatitis and liver cirrhosis patients21. The mutations G112R and Y134F were earlier reported from chronic HBV patients22. One of the samples was detected with D144E, which has also been reported earlier2324. The other mutations detected in the study were reported earlier to be associated with diagnostic or immune escape25262728.
In conclusion, our results showed an overall reduction in the pool of HBsAg carriers because of the vaccination which also helped in reducing the HBsAg carrier rate among the non-vaccinated individuals probably due to an increase in herd immunity and reduction in the source of infection. Further studies to evaluate the long-term benefits of hepatitis B vaccination among these aboriginal are required. Controls and vaccinated subjects were not matched for gender and age was a limitation of this study and might be partially responsible for the observed difference.
Acknowledgment
The authors thank the Indian Council of Medical Research, New Delhi, for providing financial grant for the study, and the Tribal Council, the village captains and the Nicobarese volunteers of Tamaloo and Big Lapathy villages of Car Nicobar Islands for their extensive support and cooperation. The first authors (HB) acknowledges the Lady Tata Memorial Trust for providing Junior Scholarship. The authors also thank Dr P. Vijayachari, Director of RMRC (ICMR), Port Blair, Directorate of Health Services, A&N Administration and District Commissioner, Nicobar group of Islands for providing administrative support during the study, and Shri D. R. Guruprasad and Ms. Sylvia Frank for providing field assistance.
References
- Tackling the hepatitis B disease burden in India. J Clin Exp Hepatol. 2014;4:312-9.
- [Google Scholar]
- World Health Organization (WHO). Expandable programme on immunization. Hepatitis B control through immunization. Global programme for vaccines and immunization subcommittee meeting of the Scientific Advisory Group of Experts. Geneva: WHO; 1995. p. :12-16.
- [Google Scholar]
- Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200:1390-6.
- [Google Scholar]
- Epidemiology of hepatitis B infection among the Nicobarese-a mongoloid tribe of Andaman and Nicobar Islands, India. Epidemiol Infect. 2002;128:465-71.
- [Google Scholar]
- Government of India. Census 2011. Available from: http://www.censusindia.gov.in/2011census/population_enumeration.aspx
- [Google Scholar]
- Immune response to an indigenously developed hepatitis-B (Shanvac B) vaccine in a tribal community of India. Vaccine. 2002;20:3431-5.
- [Google Scholar]
- Hepatitis B vaccination in a hyper endemic tribal community from India: assessment after three years. Vaccine. 2004;23:399-403.
- [Google Scholar]
- An overview of molecular epidemiology of hepatitis B virus (HBV) in India. Virol J. 2008;5:156.
- [Google Scholar]
- Presence of hepatitis B surface antigen mutant G145R DNA in the peripheral blood leukocytes of the family members of an asymptomatic carrier and evidence of its horizontal transmission. Virus Res. 2002;90:133-41.
- [Google Scholar]
- Hepatitis B virus: predominance of genotype D in primitive tribes of the Andaman and Nicobar islands, India (1989-1999) J Gen Virol. 2003;84(Pt7):1915-20.
- [Google Scholar]
- MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731-9.
- [Google Scholar]
- Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47:289-309.
- [Google Scholar]
- Distribution of hepatitis B virus genotypes: phylogenetic analysis and virological characteristics of Genotype C circulating among HBV carriers in Kolkata, Eastern India. World J Gasteroenterol. 2006;12:5964-71.
- [Google Scholar]
- Impact of hepatitis B immunization among the Nicobarese tribe - antibody titres & seroprotection five years after vaccination. Indian J Med Res. 2014;139:427-30.
- [Google Scholar]
- Eight years of hepatitis B vaccination in Colombia with a recombinant vaccine: factors influencing hepatitis B virus infection and effectiveness. Int J Infect Dis. 2008;12:183-9.
- [Google Scholar]
- The first five years of universal hepatitis B vaccination in South Africa: evidence for elimination of HBsAg carriage in under 5-year-olds. Vaccine. 2001;19:3919-26.
- [Google Scholar]
- Coexistence of hepatitis B surface antigen (HBsAg) and heterologous subtype-specific antibodies to HBsAg among patients with chronic hepatitis B virus infection. Clin Infect Dis. 2007;44:1161-9.
- [Google Scholar]
- A serological and molecular survey of hepatitis B in children 15 years after inception of the national hepatitis B vaccination program in eastern China. J Med Virol. 2009;81:1517-24.
- [Google Scholar]
- Prevalence of hepatitis B infection among the primitive tribes of Andaman & Nicobar Islands. Indian J Med Res. 2000;111:199-203.
- [Google Scholar]
- Hepatitis B seroprevalence in Thailand: 12 years after hepatitis B vaccine integration into the national expanded programme on immunization. Trop Med Int Health. 2006;11:1496-502.
- [Google Scholar]
- Hepatitis B virus gene mutations in liver diseases: a report from New Delhi. PLoS One. 2012;7:e39028.
- [Google Scholar]
- Symptomatic hepatitis B virus (HBV) reactivation despite reduced viral fitness is associated with HBV test and immune escape mutations in an HIV-co infected patient. J Infect Dis. 2008;198:1620-4.
- [Google Scholar]
- Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190:489-92.
- [Google Scholar]
- Molecular analysis of hepatitis B virus (HBV) in an HIV co-infected patient with reactivation of occult HBV infection following discontinuation of lamivudine-including antiretroviral therapy. BMC Infect Dis. 2011;11:310.
- [Google Scholar]
- A new HBsAg screening assay designed for sensitive detection of HBsAg subtypes and variants. Intervirology. 2006;49:127-32.
- [Google Scholar]
- Viral and clinical factors associated with surface gene variants among hepatitis B virus carriers. Antivir Ther. 2007;12:1255-63.
- [Google Scholar]
- A function essential to viral entry underlies the hepatitis B virus “a” determinant. J Virol. 2009;83:9321-8.
- [Google Scholar]
- Hepatitis B surface antigen variants in voluntary blood donors in Nanjing, China. Virol J. 2012;9:82.
- [Google Scholar]






