Translate this page into:
Chikungunya outbreak in Garo Hills, Meghalaya: An epidemiological perspective
Reprint requests: Dr Siraj A. Khan, Arbovirology Group/Entomology and Filariasis Division, Regional Medical Research Centre, ICMR, (NE Region). P.O. Box # 105, Dibrugarh 786 001, Assam, India e-mail: sirajkhanicmr@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Chikungunya (CHIK) fever is a mosquito-borne disease caused by chikungunya virus (CHIKV). Chikungunya infection was first reported from India in 1963 from Kolkata. We report the serological and molecular evidence of an outbreak of chikungunya in northeast India that occurred in Tura, a hilly and forested terrain in Garo Hills district of Meghalaya.
Methods:
Blood samples (3 ml) collected from hospitalized patients during the outbreak were tested for IgM antibodies against CHIKV and followed up four months later. A repeat survey was carried out in the same area after four months from where cases had been reported. Blood samples were also collected from people with history of fever and body ache in the last four months. Persons showing IgM positivity against CHIKV in the repeat survey were followed up one and a half years later. All samples were also processed by RT-PCR assay for CHIK Envelope (E) 1 gene. Immature mosquitoes were collected, link reared and identified with standard keys. Virus incrimination studies were done on Aedes aegypti and Ae. albopictus mosquitoes collected during the survey.
Results:
Fever, headache and joint pain were the primary clinical presentations. Twenty three (35.93 %) of 64 samples reported during the outbreak were IgM positive for CHIK. Three samples showed PCR amplification. All these were IgM positive. The sequenced E1 gene revealed that the strains belonged to East Central South African (ECSA) genotype.
Interpretation & conclusions:
Field survey done after four months revealed that some individuals still had joint pain associated with episodes of headache and fever. It could be inferred that these persons might have contracted infection during the CHIK outbreak four months ago or during the intervening period which caused persistence of sequelae. ECSA genotype was found to be involved in the outbreak. Aedes albopictus was the predominant mosquito species collected during the outbreak.
Keywords
Aedes albopictus
antibodies
CHIK
chikungunya
genotype
IgM
Meghalaya
Chikungunya (CHIK) is an arthropod-borne viral infection of public health importance. The disease was first reported during an outbreak in Southern Tanzania in 19521. Since then, this disease has been reported from parts of Africa, Asia and recently from some parts of Europe2. CHIK has been reported in nearly 40 countries in the form of separate outbreaks3. The clinical symptoms of CHIK include fever, joint pain, muscle pain, headache, nausea, fatigue and rash. The joint pain is often unbearable and usually lasts for a few days to weeks. The causative organism, chikungunya virus (CHIKV), belongs to genus Alphavirus, family Togaviridae4. CHIK transmission in humans is caused by Aedes (Stegomyia) aegypti (Linnaeus) and Aedes (Stegomyia) albopictus (Skuse) mosquito species5.
CHIK was first reported from West Bengal State of India in 19636. Subsequently, a major outbreak occurred in Maharashtra in 19737 after which CHIK had disappeared till 20058. Afterwards, outbreaks occurred in Andhra Pradesh and Karnataka, spreading over to eight States of the country9. In between, suspected CHIK cases have been reported from various parts of India10. The first evidence of CHIKV infection among hospitalized patients in Assam, northeast India was reported in 2011. We report here the serological and molecular evidence of a CHIK outbreak in northeast India in a Garo tribe dominated Tura township of West Garo Hills district, Meghalaya.
Material & Methods
During November 2010, suspected cases of fever of unknown origin (FUO) were reported from Tura, West Garo Hills district of Meghalaya State, India. Clinical symptoms were suggestive of CHIKV infection. The case definition of suspected CHIKV infection was as per World Health Organization (WHO) guidelines viz. fever, arthralgia, headache and backache12. A total of 64 patients suspected to have CHIKV infection were admitted to Tura Civil Hospital, Meghalaya during November 2010. All patients were from Tura township area. Serum samples from these patients were transported under cold conditions to the Regional Medical Research Centre (RMRC), Dibrugarh, Assam, for laboratory investigations. Outbreak investigation was conducted by us as per Indian Council of Medical Research (ICMR) guidelines of 10 steps for outbreak investigation during November 201013.
Serological diagnosis: All serum samples were tested for acute phase infection for immunoglobulin M (IgM) antibodies against CHIKV using IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA) kits supplied by the National Institute of Virology (NIV), Pune, India. Tests were carried out according to manufacturer's instructions.
Virus isolation and molecular detection of circulating CHIKV strains: CHIKV isolation from 14 acute phase serum samples was attempted in one day old suckling Swiss albino mice. The mice were observed for 14 days for typical symptoms of sickness. All 64 serum samples were also screened for CHIK Envelope 1 gene by reverse transcription-polymerase chain reaction (RT-PCR) based on the protocol of Yergolkar et al14, with some modifications. Viral RNA was extracted using QIAamp viral RNA mini kit (Qiagen, Germany). Complementary DNA (cDNA) was synthesised using cDNA synthesis kit (Fermentas, USA). The primers that amplified a 294 bp product of Envelope (E) 1 gene were CHIK/E1-S 5’ TAC CCA TTC ATG TGG GGC 3’ and CHIK/E1-C 5’ GCC TTT GTA CAC CAC GATT 3’. PCR amplicons were further outsourced to Eurofins Pvt. Ltd., Hyderabad, India, for sequencing. Both forward and reverse direction of each sequence were checked and edited manually using BioEdit Sequence Alignment Editor Software (BioEdit, California, USA). Edited sequences were submitted to NCBI GENBANK and respective accession numbers obtained.
Field investigations and follow up: Following laboratory confirmation of CHIK outbreak, a repeat field survey was undertaken after four months in April 2011 to assess the prevailing disease status. The CHIK positive cases were followed up and interviewed for sequelae. Blood samples (3 ml) were collected from other people in the neighbourhood who were complaining of joint pain associated with fever episodes. Following this, a second survey was done in January 2013 for the same patients after an interval of one and a half years. All samples were tested by IgM MAC ELISA kits (NIV, Pune). Long-term sequelae and other associated symptoms were recorded in a pre-tested and validated standard questionnaire developed by us. Ethical approval for carrying out the survey was obtained from RMRC, Dibrugarh Institutional Ethics Committee.
An entomological survey was undertaken by RMRC, Dibrugarh to identify the vector/s involved to plan vector control and disease containment strategies. As part of mosquito surveillance programme, solid waste containers, viz. bamboo stumps, metal and plastic wares, cans, flower pots, discarded automobile tyres, etc. holding water, as well as domestic water storage containers were searched for immature stages (larvae and pupae) of mosquitoes. Immature mosquitoes were link reared and on emergence to adults, were identified using standard mosquito identification keys15.
Statistical analysis: Data were described by time, place and person by using frequency distribution of clinical symptoms, age group and gender, period of illness as well as spatial distribution of cases in the outbreak areas. Percentage frequency of clinical symptoms, epidemic curve values and attack rates were calculated by using SPSS 13 software (SPSS, Inc., Chicago, USA). The analysis was done by comparing suspected versus laboratory confirmed cases. The categorical data were analysed using chi-square test and difference in continuous data by independent t test. The container preference of Aedes larvae was assessed by calculating breeding preference ratio16.
Results
A total of 64 patients from Tura township area were admitted to the Tura Civil Hospital during November, 2010. Of the 64 samples collected from the hospitalized patients, 23 (35.93%) were IgM ELISA positive. The common clinical presentations were fever (70%), body ache (39.13%), joint pain (30.43%) and headache (26.1%). Male patients comprised 57 per cent (n=36) of positive cases. The positive patients were from all age groups (range 12- 65 yr) with a median age of 32 yr. However, no significant association was noticed with age or gender. The distribution of CHIK cases over time gave a step ladder pattern epidemic curve (Figure). The positive cases were concentrated in four areas- Nakham Bazar, Lower Hawakhana, Rishipara and Tura Bus stand of the Tura Township which had a population of about 18000. The attack rates of CHIK cases showed that males (0.14%) suffered more than females (0.11%). The attack rate of affected population was 0.13 per cent.
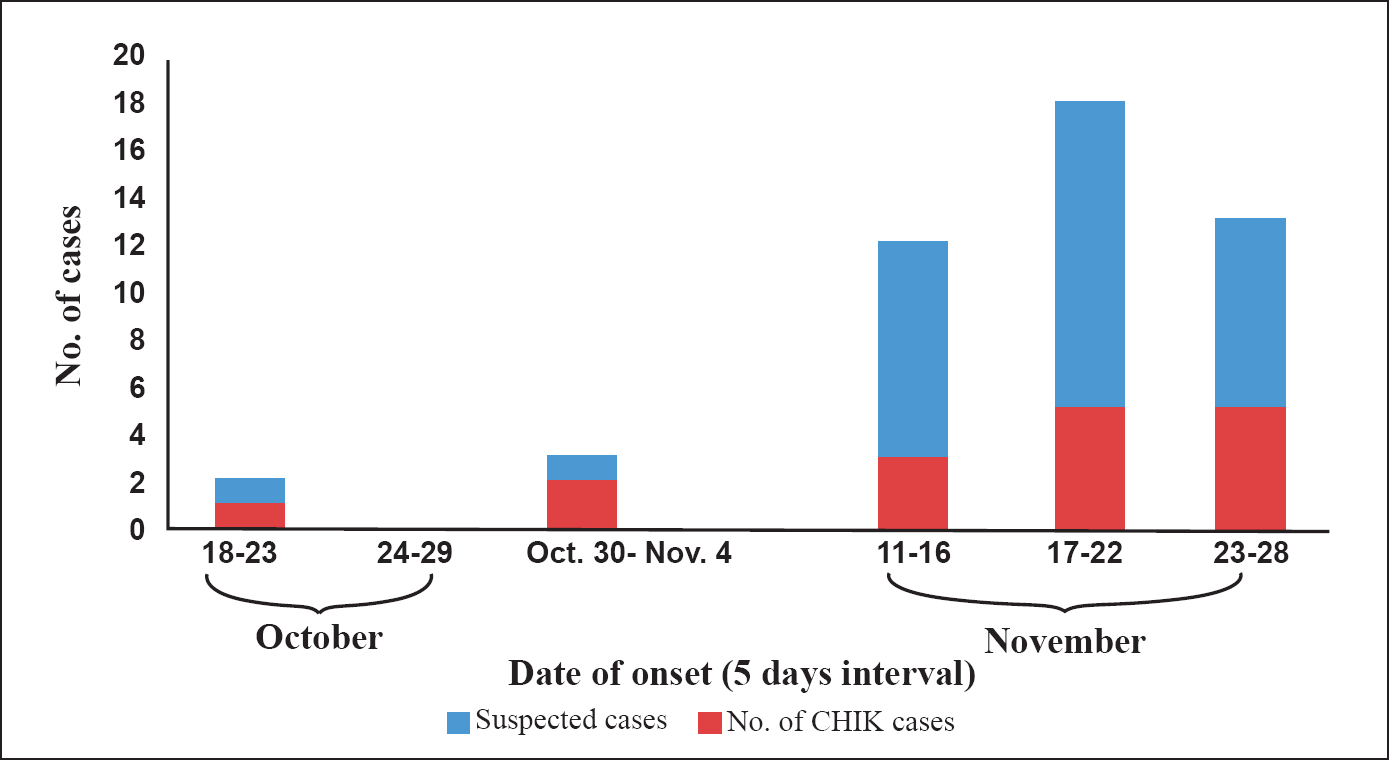
- Epidemic curve showing the incidence of CHIK cases during the outbreak in Tura, Meghalaya during 2010.
CHIKV isolation and molecular typing of circulating CHIKV isolates: No CHIKV isolate was obtained in the present study. PCR amplification was detected in 3 of 64 samples. The sequences were submitted to NCBI GENBANK (Gen Bank accession no. KF985235.1, KM058063 and KF840729.1) All three strains were placed within East Central South African (ECSA) genotype.
Human epidemiology and vector surveillance-investigations: Ten of 23 positive cases (43%) could be traced during a repeat follow up after four months of the outbreak. Persistence of joint pain was reported in all these cases. At this point of time, 110 blood samples from persons complaining of past or persisting episodes of fever and body ache from the surrounding areas of the CHIK cases were also collected. Of these, 24 (21.81%) were found positive for CHIK IgM antibodies. These cases were followed up after one and a half years to investigate long-term sequelae and persistence of IgM antibodies. Eleven of the 24 CHIK IgM positive cases could be traced at follow up (46%). None complained of any sequelae.
During the entomological survey Ae. albopictus and Ae. aegypti mosquitoes were found both in immature as well as adult collections. The most common habitats for Aedes breeding were discarded automobile tyres, mostly stored in the open in tyre repairing shops and huge solid waste scrape dump yards in the vicinity of the bus stand and market area of Tura township; bamboo stumps in the residential localities and discarded plastic containers around houses. Of the 60 households from the area surveyed, 220 containers were searched; of which, 105 had Aedes larvae. The container index (CI) and Breteau index (BI) for Ae. albopictus and Ae. aegypti are summarized in Table I. The most efficient containers in terms of breeding of Ae. albopictus were fencing bamboo stumps followed by dumped glass bottles. Ae. aegypti mosquitoes bred efficiently in tyres and cement water reservoirs. (Table II).
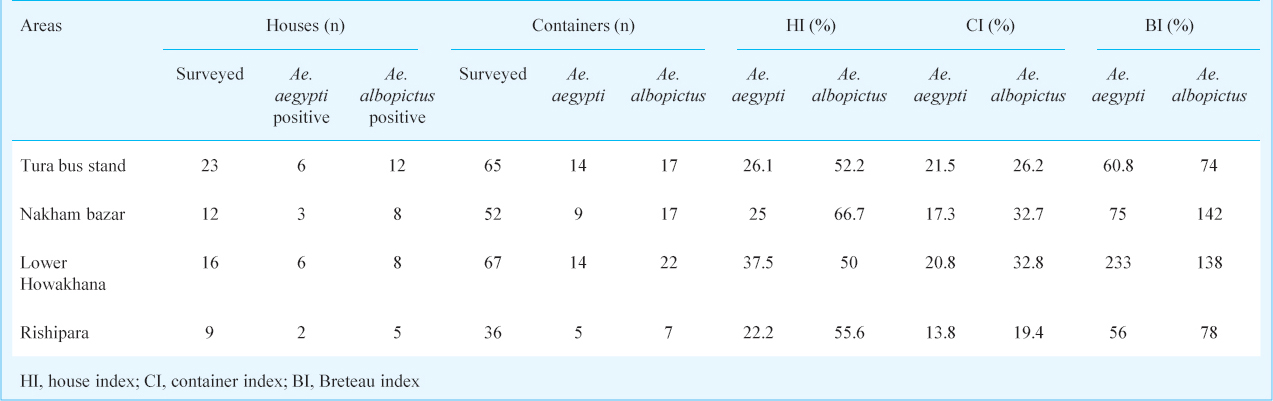
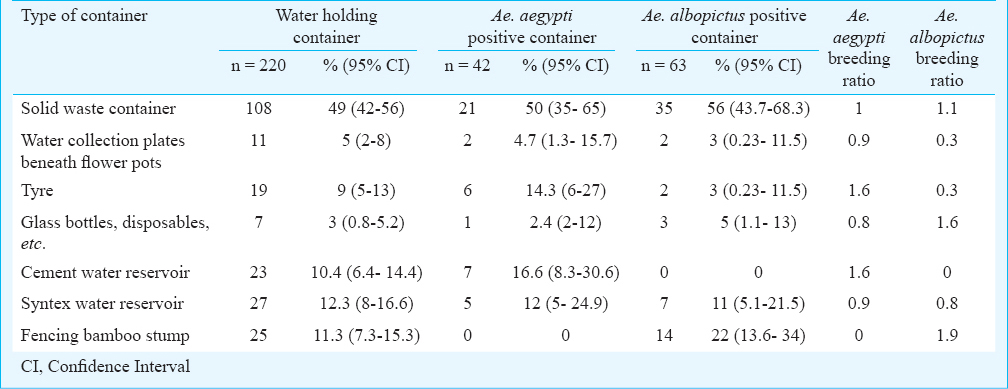
Discussion
This study reports the first CHIK outbreak in Northeast India that occurred in the State of Meghalaya in a Garo hill tribe dominated Tura Township in West Garo Hill district in 2010. The detection of IgM antibodies specific for CHIKV in acute-phase serum specimens was considered to be confirmatory17. Diagnosis of CHIK was based on two important signs in acute phase: fever and arthralgia having a specificity of 99.6 per cent and positive predictive value of 84.6 per cent18. The typical feature of CHIK includes severe joint pain in almost all cases. Similar presentation was observed in our study. Most infections completely resolve within weeks or months but there are reports of CHIK fever-induced arthralgia persisting for several years19. Persistence of CHIK typical clinical presentations- fever and arthralgia were noticed in 40 per cent positive cases after four months of infection.
The positive cases were clustered in four areas- Nakham Bazar, Lower Hawakhana, Rishipara and Tura Bus stand of Tura Township. Majority of people residing in these areas were non tribals. Epidemic curve with a step ladder pattern indicated a mosquito borne disease with an extrinsic and intrinsic period involving 10-19 days20. Tyres, water collection plates beneath flower pots, fencing bamboo stumps, reservoirs and uncovered water storage containers in addition to extensive solid waste dump yards were identified as major breeding habitats for the Aedes vector species in the affected areas. Subsequently, information, education and communication (IEC) activities combined with massive malathion fogging and Aedes breeding habitat elimination drives in the affected and adjoining areas were initiated by the Meghalaya State Health machinery that helped in checking any further spread of the disease within a span of 40 days. No new case was reported beyond November 28, 2010. During our field survey four months post outbreak, 110 people from the same locality complained of suffering from joint pain associated with fever episodes during the last four months. Twenty four of them were found to be CHIK IgM positive. These cases would probably have contacted the CHIKV infection during the outbreak and CHIK IgM is known to persist for as long as 22 months21. These positive cases were followed up after 18 months. However, no significant residual clinical presentations or sequelae were observed.
The partial E1 gene sequences demonstrated that all three CHIKV strains from Meghalaya belonged to ECSA genotype. There are three distinct genotypes of CHIKV: Asian, ECSA and Western African. The ECSA genotype has been the dominant strain throughout Asia and the islands and countries in the Indian Ocean over the last decade22. CHIKV is spread by both Ae. aegypti and Ae. albopictus species. However, in a separate study carried in Malaysia, it was observed that ECSA genotype infected Ae. albopictus at a higher rate than Ae. aegypti23. The BI threshold for predicting CHIK transmission in India is not available. However, BI higher than 5 is the threshold for dengue transmission24. It has been reported that ECSA genotype has replaced the earlier circulating Asian genotype in parts of India25. Identification of chikungunya virus ECSA genotype in the present outbreak is in conformity to the above observation.
The persistence of CHIKV and regular occurrence of outbreaks in south Asia is a major cause of concern. The Northeastern region of India shares international borders with Bangladesh, Bhutan, China, Myanmar and Nepal. Of these, Bangladesh lies at close proximity with Tura, Meghalaya. CHIK outbreaks were reported from Bangladesh in 2008 (Rajshahi district), 2009 (Pabna district)26. The Tura outbreak occurred in 2010, further followed by CHIK outbreaks in Bangladesh in Chapainababganj and Dhaka districts in 2012 and again in Dhaka in 20131927. However, no other CHIK outbreak has been reported from anywhere in Northeast India since 2010 till date. The region has been recognized as one of the hot spots of biodiversity in the world and a high prevalence of Aedes species mosquitoes throughout the entire Northeast India has been reported28. This poses a threat of occurrence of CHIK in newer areas in future. Thus, public awareness activities combined with efforts to eliminate the identified risk factors for Aedes breeding need to be undertaken to prevent any chikungunya outbreak in this region in future.
Acknowledgment
The authors acknowledge the Directorate of Health Services, Meghalaya State and the District Medical Officer and District Malaria Officer, West Garo Hill District for their support and help during the study, and thank Dr Sujoy Hazarika of Tura Civil Hospital, West Garo Hills, Meghalaya, for initial clinical diagnosis of patients. Technical support provided by N.K. Baruah and P. Doloi in entomological surveys and follow up of cases is acknowledged.
References
- World Health Organization. Chikungunya Media Centre. Available from http://www.who.int/mediacentre/factsheets/fs327/en/
- [Google Scholar]
- Centers for Disease control and Prevention. Chikungunya virus. Available from http://www.cdc.gov/chikungunya/
- [Google Scholar]
- World Health Organization. Dengue Control-Chikungunya. Available from: http://www.who.int/denguecontrol/arbo-viral/other_arboviral_chikungunya/en/
- [Google Scholar]
- Chikungunya virus infection of cell lines: Analysis of the East, Central and South African lineage. PLoS One. 2012;7:e31102.
- [Google Scholar]
- Chikungunya fever: An epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942-8.
- [Google Scholar]
- Virological investigation of the epidemic of haemorrhagic fever in Calcutta: isolation of three strains of Chikungunya virus. Indian J Med Res. 1964;52:676-83.
- [Google Scholar]
- Epidemiological investigations of chikungunya epidemic at Barsi, Maharashtra state, India. J Hyg Epidemiol Microbiol Immunol. 1979;23:445-51.
- [Google Scholar]
- Emergence of chikungunya virus in Indian subcontinent after 32 years: a review. J Vector Borne Dis. 2006;43:151-60.
- [Google Scholar]
- Chikungunya fever: the resurgence and epidemiological pattern in western India. Natl J Med Res. 2013;3:159-61.
- [Google Scholar]
- Epidemiology, clinical manifestations, and diagnosis of chikungunya fever: lessons learned from the re emerging epidemic. Indian J Dermatol. 2010;55:54-63.
- [Google Scholar]
- First evidence of chikungunya virus infection in Assam, Northeast India. Trans R Soc Trop Med Hyg. 2011;105:355-7.
- [Google Scholar]
- World Health Organization (WHO) In: Guidelines on clinical management of chikungunya fever. Geneva: WHO; 2008.
- [Google Scholar]
- Indian Council of Medical Research, New Delhi. Outbreak investigation checklist: 10 steps to follow, 10 pitfalls to avoid. Available from: http://www.nie.gov.in/images/leftcontent_attach/3.A.7.PittOutb_153.pdf
- [Google Scholar]
- Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580-3.
- [Google Scholar]
- Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmission. Zootaxa. 2004;78:239-41.
- [Google Scholar]
- A study on container breeding mosquitoes with special reference to Aedes (Stegomyia) aegypti and Aedes albopictus in Thiruvananthapuram district, India. J Vector Borne Dis. 2014;51:27-32.
- [Google Scholar]
- Clinical features and molecular diagnosis of chikungunya fever from south India. Clin Infect Dis. 2008;46:1436-42.
- [Google Scholar]
- Prospective study of chikungunya virus acute infection in the Island of La Réunion during the 2005-2006 outbreak. PLoS one. 2009;4:e7603.
- [Google Scholar]
- Chikungunya - an emerging infection in Bangladesh: a case series. J Med Case Rep. 2014;8:67.
- [Google Scholar]
- Dengue outbreak in an Indo-Myanmar border areas: epidemiological aspects and risk factors. Trop Biomed. 2013;30:451-8.
- [Google Scholar]
- Evidence for endemic chikungunya virus infections in Bandung, Indonesia. PLoS Negl Trop Dis. 2013;7:e2483.
- [Google Scholar]
- The threat of chikungunya in Oceania. Western Pac Surveill Response J. 2013;4:8-10.
- [Google Scholar]
- Genotypic and phenotypic characterization of chikungunya virus of different genotypes from Malaysia. PLoS One. 2012;7:e50476.
- [Google Scholar]
- Molecular and Virological Investigation of a Focal Chikungunya Outbreak in Northern India. Scientific World J. 2013;2013:1-6.
- [Google Scholar]
- Travel health: news item. Update on chikungunya in Bangladesh. Available from: http://vhi.exodus.ie/news.asp?id=59095
- [Google Scholar]
- An updated checklist of species of Aedes and Verrallina of northeastern India. J Am Mosq Control Assoc. 2010;26:135-40.
- [Google Scholar]






