Translate this page into:
Antimicrobial susceptibility pattern of vancomycin resistant enterococci to newer antimicrobial agents
*For correspondence: varshagupta_99@yahoo.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Enterococci are recognized as opportunistic pathogens and are natural inhabitants of the oral cavity, gastrointestinal tract (GIT) and the female genital tract in both humans and animals1. They have emerged as important nosocomial pathogens2. There are two main species - Enterococcus faecalis and E. faecium responsible for human enterococcal infections3. The most frequent infections caused by these organisms include urinary tract infections, intra-abdominal and intra-pelvic abscesses. These are increasingly being isolated from bacteraemia and meningitis cases mainly from hospitalized patients4.
The emergence of resistance to the most common anti-enterococcal antibiotics which include the β-lactam antibiotics like ampicillin, aminoglycosides and most importantly glycopeptides like vancomycin besides being inherently resistant to many others like cephalosporins and clindamycin has made the treatment of these infections a real challenge for clinicians5. With the increase in emergence of resistance in enterococci to vancomycin, treatment of these infections has become difficult especially in serious infections6. Since options for the treatment of patients with vancomycin resistant enterococci (VRE) are very limited, this study was aimed to assess the potential usefulness of compounds, which have come into recent use. Newer antibiotics such as linezolid, daptomycin and tigecycline have shown good in vitro activity against VRE7. Quinupristin-dalfopristin (Q/D) is another agent that has potent in vitro activity against E. faecium but poor activity against E. faecalis8.
This study was conducted in the department of Microbiology, Government Medical College and Hospital, Chandigarh, India. In this study, the in vitro activity of vancomycin, teicoplanin, tigecycline, daptomycin, linezolid and quinupristin/dalfopristin has been evaluated against 75 non-repeat clinical isolates of vancomycin resistant E. faecalis (60) and E. faecium (15) by MIC (minimum inhibitory concentration) testing with Epsilometer test (E-test) method (E-test, AB Biodisk, Solna, Sweden). These 75 VRE isolates were obtained over a period of three years (2009-2011) from various samples namely, urine (56), blood (8) and pus (11). All these isolates were identified as Enterococcus according to standard methods and species identification was done using the conventional test scheme9. Initially the vancomycin resistant isolates were collected based on disc diffusion results as per Clinical and Laboratory Standards Institute (CLSI) guidelines using vancomycin disc (30 µg)10. All culture media, antibiotics discs and standard strains of bacteria used in this study were procured from Hi-media Laboratories Pvt. Ltd., Mumbai, India. E. faecalis ATCC 29212 and E. faecalis ATCC 51299 were used for quality control. E-test strips of vancomycin, teicoplanin, tigecycline, daptomycin, linezolid and quinupristin/dalfopristin were obtained from AB BioDisk, Solna, Sweden. E-test to daptomycin was done on Mueller-Hinton agar supplemented with 50 mg/l calcium (Difco, USA) due to daptomycin's dependence on calcium. MIC values were interpreted according to the CLSI guidelines10 except for tigecycline for which EUCAST was followed11.
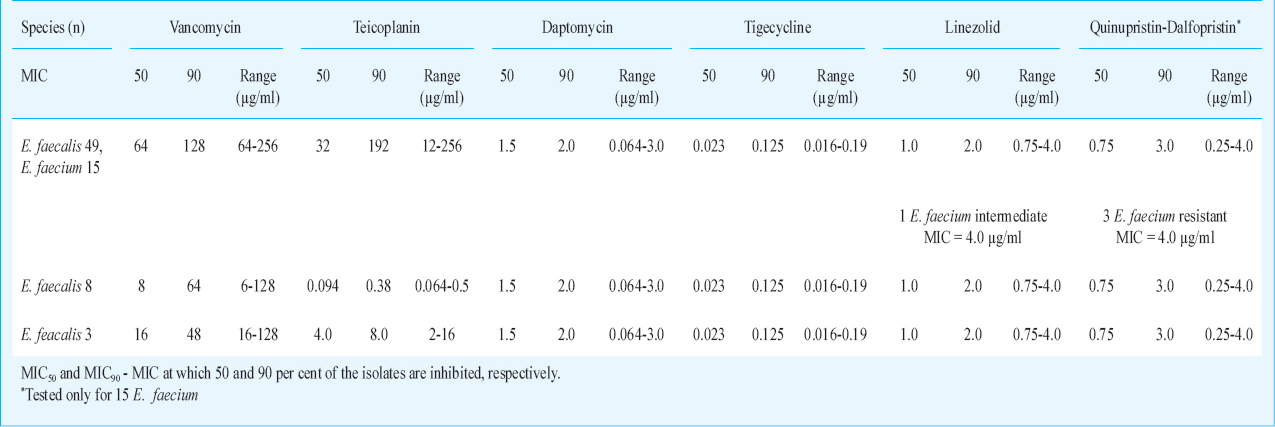
Based on the MIC values for glycopeptides - vancomycin and teicoplanin, in our study majority of strains (49 E. faecalis and 15 E. faecium) belonged to the vanA resistance phenotype having high level vancomycin and teicoplanin resistance (MIC values being in the range of 64 to 256 µg/ml). We had eight E. faecalis isolates of van B type having variable levels of vancomycin resistance but were susceptible to teicoplanin (MIC values being in the range of 64 to 128 µg/ml for vancomycin and 0.064-0.50 µg/ml for teicoplanin). Three E. faecalis isolates had vancomycin MIC as 64 and teicoplanin MIC to be 4.0, 4.0, and 8.0 µg/ml, so probably these were van D type. Van D-type strains are characterized by moderate levels of resistance to both vancomycin and teicoplanin. Earlier reports from India have shown mainly vanA and vanB phenotypes12. The phenotypic classification of VRE in to various types is solely based on the vancomycin and teicoplanin breakpoints and is not a reliable method, and has some limitations also. Earlier report has shown that mutations in van B strains can exhibit resistance to teicoplanin and such strains become phenotypically indistinguishable from van A resistant phenotypes13. These strains need to be confirmed by molecular characterization.
A number of relatively new agents that possess clinical data and ultimately clinical utility in the treatment of more serious infections due to VRE have been studied7. There is limited information reported from India14. In our study, all our isolates had MIC for linezolid within susceptibility range, MIC values ≤ 2 µg/ml except for one E. faecium showing linezolid MIC to be 4 µg/ml which is intermediate susceptibility. A study from India has shown 100 per cent susceptibility of VRE to linezolid14. Yasliani et al15 reported two isolates of E. faecium resistant to linezolid from Tehran with MIC 32 µg/ml. Susceptibility of VRE to linezolid was shown to decrease to 83 per cent six months after inclusion of linezolid on the hospital antibiotic policy16. A study from India showed daptomycin to be the most active agent against VRE, highlighting the importance of the drug as an excellent therapeutic option17. We also found all VREs to be 100 per cent susceptible to daptomycin (MIC ≤ 4.0 µg/ml). A surveillance of US hospitals showed that more than 99.5 per cent of VRE isolates were susceptible to daptomycin18192021. But empiric daptomycin therapy for VRE infections should be used with caution and be based on susceptibility data19. Daptomycin resistance in enterococci was observed in a previously sensitive E. faecalis isolate, while on therapy2122. Quinupristin-dalfopristin is a streptogramin antibiotic active only against E. faecium. Resistance to quinupristin/dalfopristin has been reported in 1.3-2.4 per cent of patients with VRE23. We found three isolates of E. faecium resistant to Q/D(MIC=4.0 µg/ml) (Table). Quinupristin/dalfopristin is advocated for vancomycin-resistant E. faecium infections in critically ill patients with serious underlying diseases23. In our study, all enterococcal isolates were found to be susceptible to tigecycline (MIC≤ 0.25 µg/ml), which was in agreement with a study from south India24. Cases of E. faecalis isolates with MIC 6 µg/ml for tigecycline have also been described25. A study from Korea showed high resistance to all the newer drugs and out of all tigecycline was found to be an effective drug26. Of all these antibiotics considered, daptomycin is the only bactericidal drug while linezolid, tigecycline and quinupristin/dalfopristin are bacteriostatic drugs. All have their side effects also27. Further, linezolid, tigecycline, and daptomycin, have activity against both vancomycin-resistant E. faecalis and E. faecium, whereas quinupristin-dalfopristin has activity against E. faecium only. Daptomycin is found to be inhibited by pulmonary surfactant so should not be used for pneumonias28.
Vancomycin still remains the mainstay of treatment for serious enterococcal infections, if the strain is found susceptible. However, with the emergence of resistance to vancomycin other antibiotics like linezolid, quinupristin-dalfopristin, tigecycline and daptomycin can also be considered. The data on local patterns of susceptibility of VRE to newer antimicrobial agents can help in guiding the treatment of these pathogens.
References
- Enterococci: new aspects of an old organism. Proc Assoc Am Physicians. 1999;111:328-34.
- [Google Scholar]
- The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol. 2012;10:266-78.
- [Google Scholar]
- Clinical prevalence, antimicrobial susceptibility, and geographic resistance patterns of enterococci: results from the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001;32(Suppl 2):S133-45.
- [Google Scholar]
- Enterococcal infections & antimicrobial resistance. Indian J Med Res. 2008;128:111-21.
- [Google Scholar]
- Enterococcal infections with special reference to phenotypic characterization & drug resistance. Indian J Med Res. 2004;119(Suppl):22-5.
- [Google Scholar]
- Current concepts in antimicrobial therapy against select Gram-positive organisms: methicillin-resistant Staphylococcus aureus, penicillin-resistant pneumococci, and vancomycin-resistant enterococci. Mayo Clin Proc. 2011;86:1230-43.
- [Google Scholar]
- Antimicrobial activity of quinupristin-dalfopristin (RP 59500, synercid) tested against over 28,000 recent clinical isolates from 200 medical centers in the United States and Canada. Diagn Microbiol Infect Dis. 1998;31:437-51.
- [Google Scholar]
- Enterococcus. In: Murray BE, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, eds. Manual of clinical microbiology. Washington (DC): American Society for Microbiology; 2007 Press; p. :430-42.
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 20th Informational Supplement, vol. 30, M100-S20. Wayne, Pa, USA: CLSI; 2010.
- [Google Scholar]
- European committee on antimicrobial Susceptibility testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters, version 1.1. Stockholm, Sweeden: Eucast; 2010.
- [Google Scholar]
- Epidemiology and molecular characterization of vancomycin resistant Enterococci isolates in India. Scand J Infect Dis. 2007;39:662-70.
- [Google Scholar]
- In vivo development of teicoplanin resistance in a VanB Enterococcus faecium isolate. J Infect Dis. 1993;167:1224-7.
- [Google Scholar]
- In vitro activity of daptomycin & linezolid against methicillin resistant Staphylococcus aureus & vancomycin resistant enterococci isolated from hospitalized cases in Central India. Indian J Med Res. 2013;137:191-6.
- [Google Scholar]
- Linezolid vancomycin resistant Enterococcus isolated from clinical samples in Tehran hospitals. Indian J Med Sci. 2009;63:297-302.
- [Google Scholar]
- Clinical-use-associated decrease in susceptibility of vancomycin-resistant Enterococcus faecium to linezolid: a comparison with quinupristin-dalfopristin. Antimicrob Agents Chemother. 2004;48:3583-5.
- [Google Scholar]
- In vitro activity of daptomycin against Staphylococcus aureus and vancomycin-resistant Enterococcus faecium isolates associated with skin and soft tissue infections: first results from India. Diagn Microbiol Infect Dis. 2009;65:196-8.
- [Google Scholar]
- Antimicrobial susceptibility of gram-positive bacteria isolated from US medical centers: results of the Daptomycin Surveillance Program (2007-2008) Diagn Microbiol Infect Dis. 2009;65:158-62.
- [Google Scholar]
- Emergence of daptomycin-resistant VRE: experience of a single institution. Infect Control Hosp Epidemiol. 2011;32:391-4.
- [Google Scholar]
- A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev. 2013;26:759-80.
- [Google Scholar]
- Genetic basis for in vivo daptomycin resistance in Enterococci. N Engl J Med. 2011;365:892-900.
- [Google Scholar]
- Optimizing therapy for vancomycin-resistant enterococci (VRE) Semin Respir Crit Care Med. 2007;28:632-45.
- [Google Scholar]
- Quinupristin/Dalfopristin therapy for infections due to vancomycin-resistant Enterococcus faecium. Clin Infect Dis. 2000;30:790-7.
- [Google Scholar]
- Evaluation of tigecycline activity in clinical isolates among Indian medical centers. Indian J Pathol Microbiol. 2010;53:734-7.
- [Google Scholar]
- Tigecycline-resistant Enterococcus faecalis associated with omeprazole use in a surgical patient. J Antimicrob Chemother. 2012;67:1806-7.
- [Google Scholar]
- Antimicrobial activity of mupirocin, daptomycin, linezolid, quinupristin/dalfopristin and tigecycline against vancomycin-resistant enterococci (VRE) from clinical isolates in Korea (1998 and 2005) J Biochem Mol Biol. 2007;40:881-7.
- [Google Scholar]
- Effects of prior effective therapy on the efficacy of daptomycin and ceftriaxone for the treatment of community-acquired pneumonia. Clin Infect Dis. 2008;46:1142-51.
- [Google Scholar]





