Translate this page into:
Use of maggot therapy for treating a diabetic foot ulcer colonized by multidrug resistant bacteria in Brazil
Reprint requests: Dr Renato Motta Neto, Department of Microbiology & Parasitology, Biosciences Center, Federal University of Rio Grande Do Norte, Av. Senador Salgado Filho, 3000, Candelaria, Natal, RN, Brasil e-mail: renato@cb.ufrn.br
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This study reports the efficacy of maggot therapy in the treatment of diabetic foot ulcer infected with multidrug resistant microorganisms. A 74 year old female patient with diabetes for over 30 years, was treated with maggot therapy using larvae of Chrysomya megacephala. The microbiological samples were collected to evaluate aetiology of the infection. The therapy done for 43 days resulted in a reduction of necrosis and the ulcer's retraction of 0.7 cm2 in area. Analysis of the bacteriological swabs revealed the presence of Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa. Further studies need to be done to confirm the role of maggot therapy in wound healing using a large sample and a proper study design.
Keywords
Chrysomya megacephala
diabetic foot ulcer
Klebsiella pneumoniae
Pseudomonas aeruginosa
Maggot therapy is known to be used in chronic wounds to remove necrotic tissue, stimulate granulation tissue formation and kill bacteria12. In diabetic foot ulcers with the problem of bacterial resistance, this therapy has been used as an alternative treatment of these ulcers. We report here a case study in Brazil using maggot therapy for treating chronic ulcers infected with multidrug-resistant bacteria in a diabetic patient.
A 74 year old female patient having diabetes for over 30 years, reported to the Surgical Clinic Department, University Hospital Onofre Lopes (HUOL)- University Federal do Rio Grande do Norte, Brazil, with a foot ulcer in August 2012. The second instar larvae of the Chrysomya megacephala (Diptera: Calliphoridae) were used in this study for maggot therapy and the maintenance and eggs disinfection processes were performed using procedures recommended by Marcondes3. The larvae were obtained from established colonies in the insectory of Laboratory of Insect and Vectors, Department of Microbiology and Parasitology, Biosciences Center, Federal University of Rio Grande do Norte. The larvae were removed from the medium, washed with sterile water and transferred to a sterile container. The technique of maggot therapy consisted of an initial wash with saturated sodium chloride followed by collection of clinical specimens for microbiological analysis and application of the free-range larvae (5 per cm2 of tissue compromised) directly at the ulcer. The clinical specimen was sent in Stuart transport medium (Difco Laboratories, USA) to Mycobacteria Laboratory, department of Microbiology and Parasitology, Federal University of Rio Grande do Norte. The processing of clinical specimens was performed using procedures and culture media recommended by Murray et al4. Biochemical tests were performed for bacteria identification at species level. To perform the antimicrobial sensibility test (AST), the Kirby-Bauer's agar disk diffusion method was used5. The bacterial strains that showed the multidrug resistance pattern were subjected to genotypic analysis to confirm the presence of genes encoding TEM, SHV, and CTX-M6. Strains of Escherichia coli (ATCC 25922) and Klebsiella pneumoniae (ATCC 700603) were included as controls.
The study was approved by the ethics committee of the Federal University of Rio Grande do Norte, Brazil. Six biological dressings were done with an interval of every 48 h. Before therapy, an extension of tissue impairment of 8.4 cm2 was obtained (Fig. A) and after two weeks of treatment (14 days) a retraction of the ulcer 0.7 cm2 was observed (Fig. B). At the end of treatment (43 days) the ulcer's surface area was occupied by granulation tissue (Fig. C). The maggot therapy was found to be an effective and inexpensive method of debridement, providing a rapid acceleration in the process of wound healing78. Importantly, the effect of using the debridement maggot therapy occurs not only by the physical activity of larvae (by their mandibles) but also by secretions and excretions such as trypsin, collagenase, and chymotrypsin, showing that the lytic activity is able to promote the dissolution of necrotic tissue. This therapy also eliminated multidrug resistant microorganisms (K. pneumoniae, E. coli, Pseudomonas aeruginosa) present in the wound, as reported earler9. The SHV and CTX-M genotypes were observed only in the K. pneumoniae isolates. These results indicated the presence of ESBL (extended-spectrum beta-lactamase) in bacteria. Bexfield et al10 demonstrated the efficacy of these compounds against strains of methicillin-resistant Staphylocccus aureus and other bacteria. Several peptides produced in whole body extract of maggots with high antimicrobial activity as well as compounds present in their haemolymph have been shown to have activity against P. aeruginosa11.
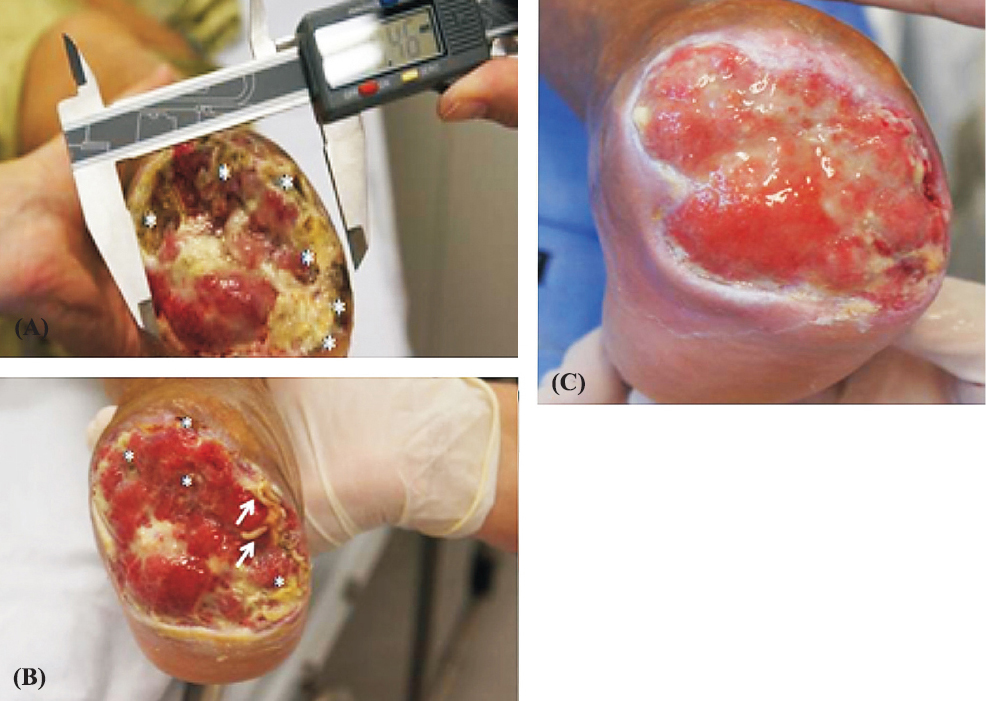
- Patient's foot ulcer before and after the maggot therapy. (A). Measurement of the extent of necrosis and application of treatment (day 1). Asterisks represent areas of tissue necrosis. (B). Patient's foot ulcer image during treatment with maggot therapy (day 14). The asterisks represent areas of tissue necrosis and the arrows indicate the larvae of Chrysomya megacephala. (C). Patient's foot ulcer image after treatment with maggot therapy (day 43).
In conclusion, considering the role of maggot therapy in wound healing, particularly in diabetic ulcer and its low cost compared with the other usual treatments, there is a need to evaluate its safety and efficacy on a large number of patients.
References
- Medicinal Maggots: an ancient remedy for some contemporary afflictions. Annu Rev Entomol. 2000;45:55-81.
- [Google Scholar]
- Use of maggot therapy for treating a diabetic foot ulcer colonized by multidrug resistant bacteria in Brazil. 2006
- [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 23rd informational supplement. 2013
- [Google Scholar]
- Prevalence of extended spectrum beta-lactamases producing microorganisms in noscomial patients and molecular characterization of the shv type isolates. Braj J Microbiol. 2010;41:278-82.
- [Google Scholar]
- Maggot debridement therapy in the treatment of chronic wounds in a military hospital setup in Turkey. Dermatology. 2005;210:115-8.
- [Google Scholar]
- Degradation extracellular matrix components by defined proteinases from the green bottle larva Lucillia sericata used for the clinical debridement of non-healing wounds. Br J Dermatol. 2003;148:14-23.
- [Google Scholar]
- Venous ulceration contaminated by multi-resistant organisms: larval therapy & debridement. J Wound Care. 2013;22(Suppl 10):s27-30.
- [Google Scholar]
- Detection and partial characterization of two antibacterial factors from the excretions/secretions of the medicinal maggot Lucilia sericata and their activity against methicillin-resistant Staphylococcus aureus (MRSA) Microbes Infect. 2004;6:1297-304.
- [Google Scholar]
- Antibacterial properties of whole body extracts and haemolymph of Lucilia sericata maggots. J Wound Care. 2007;16:123-7.
- [Google Scholar]






