Translate this page into:
Survival of diurnally sub periodic Wuchereria bancrofti in Downsiomyia nivea (Diptera: Culicidae): a density dependent factor from Andaman & Nicobar Islands
Reprint requests: Dr A.N. Shriram, Scientist B, Regional Medical Research Centre (ICMR) Post Bag No.13, Port Blair 744 101, Andaman & Nicobar Islands, India e-mail: shriraman@icmr.org.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
In India, diurnally sub periodic Wuchereria bancrofti transmitted by Downsiomyia nivea is prevalent only in the Nicobar district of Andaman and Nicobar Islands. The ongoing LF elimination programme aims at transmission interruption by bringing down the microfilarie (mf) load in the community, which has implication on the parasite load in mosquito vector. Therefore, understanding density dependent constraints on transmission assumes significance from control perspective. The present study was undertaken in Teressa Island to understand the density dependent parasite mortality and survival probability of the parasite Do. nivea.
Methods:
The entomological data collected from Teressa Island, endemic for the diurnally sub periodic form of W. bancrofti were used to examine the parasite loss and its survival up to infectivity. Patterns of parasite distribution in Do. nivea were examined.
Results:
Distribution patterns of microfilariae were found to be over dispersed in Do. nivea. The later stages of the parasite in the vector were randomly distributed. Distribution pattern of various filarial larval stages suggested that the loss of parasites occurred as development progressed and was maximal between the first and second stages. Further, both the prevalence of infection and the degree of parasite aggregation in the vector population have fallen significantly with development of parasite stage.
Interpretation & conclusions:
Results indicate the operation of parasite density dependent mortality of vectors or parasite loss or combination of both. The present study with Aedes transmitted filariasis conducted before launching LF elimination programme in the study area indicates a comparable level of parasite regulation in the vector which has similar implications on the transmission threshold. Thus, the consideration of Aedes with Culex in deriving the critical level of antigen positive for making decisions on cessation of mass drug administration (MDA) can be justified. However, with MDA aiming at reducing parasite load in the community, the operation of density dependent factor in the transmission becomes less pronounced in the subsequent rounds of MDA.
Keywords
Andaman & Nicobar Islands
density dependent
diurnally sub periodic
Downsiomyia nivea
parasite mortality
vector
Wuchereria bancrofti
Lymphatic filariasis (LF) is an important public health problem in India contributing about 40 per cent of the global burden1. The prevalence of LF in Andaman and Nicobar archipelago in India was identified as early as 19422 when Nicobar group of islands showed an infection rate of 5.8 per cent with Wuchereria bancrofti. Subsequent survey conducted in 19583 showed the prevalence of LF in both Andaman and Nicobar Islands. Diurnally sub periodic form of W. bancrofti (DspWB), transmitted by Ochlerotatus niveus (now known as Downsiomyia nivea)4 is endemic only in a small pocket of seven islands viz., Nancowry group of islands of the Nicobar district in India5. The first report on the prevalence of DspWB, transmitted by O. niveus came from Nicobar group of islands in 19745. Subsequent studies have shown its prevalence and perennial transmission in all the seven islands of Nancowry group of islands67891011.
The central inquiry into the epidemiology of vector-borne diseases is to understand whether density dependent processes operate on parasite transmission in the vector population1213. By and large this can manifest itself with increasing parasite uptake as facilitation (increase in infective larvae) or limitation (decrease in infective larvae). Both these phenomena impose a density dependent constraint on transmission. The main mechanism proposed to derive limitation includes excess mortality of the parasites and parasite induced vector mortality at high parasite densities131415.
Experimental16 and field studies17 have shown excess mortality of Culex quinquefasciatus heavily infected with W. bancrofti. Operation of such a phenomenon in sub periodic W. bancrofti and Do. nivea combination has now been studied using the distribution pattern of W. bancrofti larvae in Do. nivea to assess the existence of regulatory mechanism as reported in Cx. quinquefasciatus18.
An analysis19 on the distribution of W. bancrofti larvae in a natural population of the Cx. quinquefasciatus in an endemic region for bancroftian filariasis in Sri Lanka has shown that the distribution of microfilariae in freshly blood-fed mosquitoes did not differ significantly from that in blood samples from the human population. The results from experimental infections were used to understand the relative reduction in the proportion of vectors with heavy burden of older parasites in terms of the operation of parasite-induced host mortality. Similar observations have been made in Puducherry, an endemic region for nocturnally periodic bancroftian filariasis transmitted by Cx. quinquefasciatus20.
Under experimental conditions, it has been established that the heterogeneity of the intermediate host behaviour and aggregated spatial distribution of infective stage larvae can generate a high degree of over-dispersion of parasite numbers per host in the definitive host21. Therefore, it is important to see whether the distribution of infective stage larvae of W. bancrofti in the intermediate host (vector) is responsible for over-dispersion of parasites in the human host. Further, studies on the rate of loss of parasites during their development in the vector and the parasite-inflicted mortality in the vector population, which plays a major role in the transmission of disease, are very limited. The ongoing LF elimination programme aiming at interrupting transmission by bringing down the microfilariae load in the community, has implications on the parasite load in the vector population. Therefore, this study was carried out to understand the density dependent constraints on transmission of filariasis in Teressa island of Andaman and Nicobar Islands which has implications from the perspective of filariasis control.
Material & Methods
Study area: The Nancowry group of islands of Nicobar district (8.50-9.50 N and 930-940 E) is a small region composed of seven remotely located islands (Bompoka, Chowra, Kamorta, Katchal, Nancowry, Teressa and Trinket) with a total population of approximately 25000 people, mainly Nicobarese tribes who are at risk of acquiring W. bancrofti filarial infection. The present study was carried out between November 1999 and October 2000, in Teressa island (8° 20’ N and 93° 7’ and 93° 15’ E) in Bay of Bengal. This island has an area of 87.04 km2 and a population of 1,935 Nicobarese residing in 11 villages. The native inhabitants depend mainly on pigs for food, which they rear. For their livelihood, the people collect forest produce. All villages are surrounded by forest interspersed with coconut and arecanut groves. This island also has densely forested tropical jungles.
Mean minimum temperatures on this island ranged between 22.9°C (January) and 25.4°C (March) and mean maximum temperature 28.3°C (January) and 32.4°C (March). The Relative humidity is high and ranged between 72.9 per cent (January) and 87.0 per cent (November). Rainfall is heavy from May to November, and is influenced by both the southwest and northeast monsoons. In the other months, rainfall is generally low, with February being the driest month. The rainfall ranged between 32.7 mm (March) and 351.1 mm (May) during the study period. The soil is porous coral sand, quickly absorbing the rainwater and leaving hardly any stagnant water. Tree holes are the major breeding habitats of Do. nivea in the Nancowry group of islands7.
Sample size: Assuming that the mosquito infection rate in the study area was 3 per cent, allowing 20 per cent error with 95 per cent confidence level, the sample size for infinite population was computed as 3250. In view of constraints in sampling adult population it was decided to collect a minimum of 3500 mosquitoes during the study period from different study areas.
Mosquito collection: Human landing collections were made in five randomly selected villages of the eleven villages. Human-landing collections were carried out in all five selected villages, from dawn to dusk at monthly intervals in fixed catching stations for a one year period. A human volunteer identified from the respective villages, consented to be the bait. He sat on a raised wooden platform that formed part of the outdoor extension of the Nicobarese hut, adjoining the forest fringe, wearing his normal clothing. Mosquitoes attempting to bite his exposed body surface were collected using oral aspirators by an insect collector. During the human-landing collections, insect collectors worked in shifts, but the same person acted as bait.
The study protocol was approved by the Institutional Ethical Committee of the Regional Medical Research Center (RMRC), Port Blair. Verbal informed consent was obtained from the volunteers.
Mosquito identification and dissection: Hourly collections of mosquitoes were kept separate and brought alive to the field laboratory, anaesthetized with ether and identified using standard keys22. All the mosquitoes were dissected to determine the physiological age and infection with the filarial parasite. Ovarioles dissected out from the ovaries were examined under a compound microscope for dilatations to determine the physiologic age23. The abdomen, thorax and head of the mosquito were teased and examined under a compound microscope at 100X magnification for the presence of W. bancrofti larvae. The filarial larvae were categorized into microfilariae (mf), L1 stage (short, inactive sluggish and sausage shaped), L2 stage (longer and active compared to the stage L1) and infective stage or L3 (long, very active, relatively thin and found in any part of the mosquito body) as per the description of Sasa24. Mosquitoes with any filarial stages of the parasite were considered as infected and those with infective stage larvae (L3) as infective. The total number of different stages of larvae present in different parts of the mosquito body was recorded.
Statistical analysis: Chi square test was carried out to test the hypothesis that mosquitoes with each stage of the parasite infection are independent of age of the mosquitoes. The data on age of the mosquito and for the presence or absence of parasite stages were organized in a 4X2 Table. To assess the distribution patterns of parasites, the frequency distributions of parasite counts were constructed for each parasite stage. The trend of aggregation in parasite density was measured in terms of variance to mean ratio for different mosquito and parasite stages. The probability of survival through one day was calculated based on ‘nth’ root of proportion of parasite survival (p) of a particular stage, where ‘n’ is the total development period from mf to the stage concerned15.
Results
The data set comprised the results of the monthly man landing collections, which yielded a total of 3625 female Do. nivea for detection of the infection due to W. bancrofti and age grading, according to the number of previous egg layings (parity). There were 2,767 (76.33%) nulliparous mosquitoes. Among the parous mosquitoes, 659 (18.18%) had evidence of one contact with the host, 172 (4.74%) had two contacts, and 27 (0.74%) had three contacts. Of all these, 97 (2.68%) mosquitoes were infected with filarial larvae and 18 (0.5%) mosquitoes were with infective stage (Table I). The infection rates in cool, summer and monsoon season were 2.5, 3.2 and 2.3 per cent, respectively and there was no significant variation between seasons. The corresponding infectivity rates (0.9, 0.4 and 0.4% were also not statistically significant (χ2 =.0.262; P>0.05).
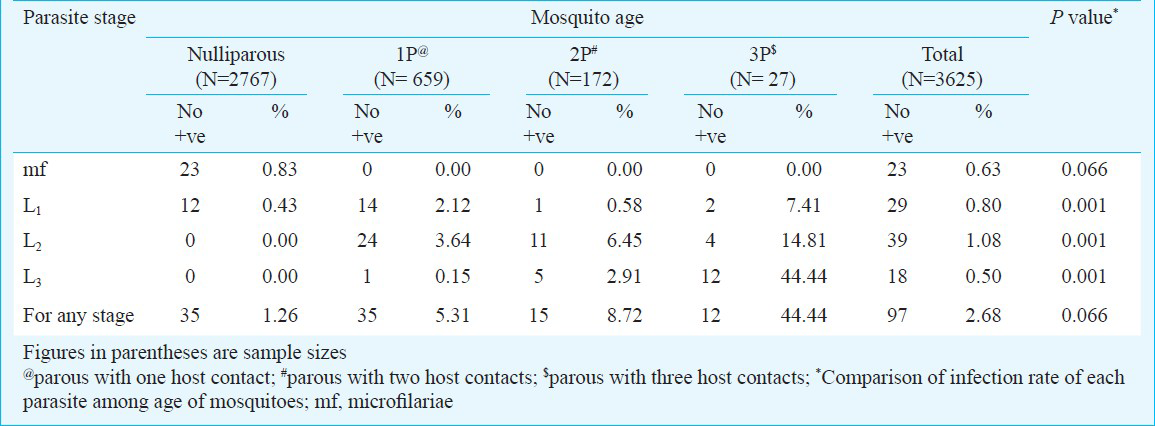
Prevalence of infection of mosquitoes with parasite stages: The sample size and number of mosquitoes positive in relation to mosquito age and infection are presented in Table I. The infection rate increased significantly with the age of the mosquitoes (P < 0.001) implying that the risk of acquiring infection from parous mosquitoes having three contacts, was about 63 times compared to that of nulliparous mosquitoes (1.0). Similarly, the infective rate showed a significantly increasing trend with mosquito age (P < 0.001) indicating that the risk of acquiring infection from parous mosquitoes with three host contacts was 526 times compared to those with 1 parous (1.0). However, mf infection rate did not differ significantly across age of mosquitoes.
Density dependent parasite mortality: The loss of parasites due to the mortality of heavily infected vectors was evident from the distribution patterns of different stages of the parasites and from the drastically reduced tail of the curve of infective stage larvae (Figure). The wide gap in between the L1 and L2 indicated that the loss of parasites in mosquitoes was highest between the L1 and L2 stages.
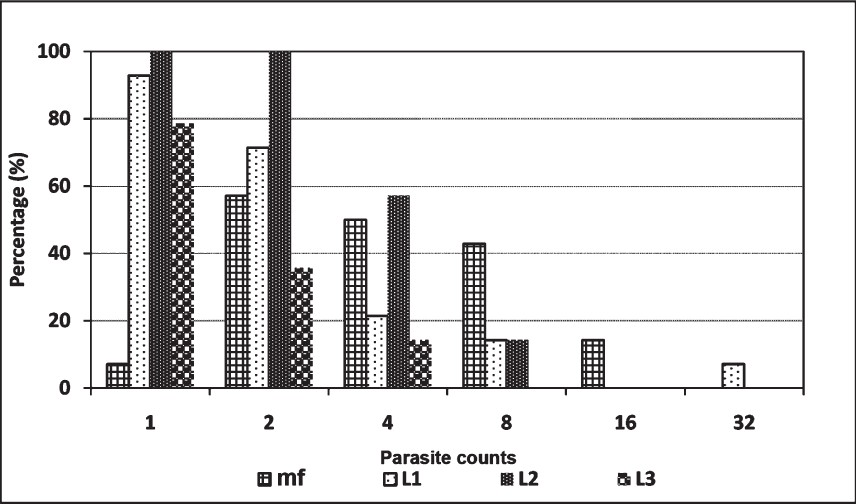
- Distribution patterns of mf, L1, L2 and L3 in Do. nivea.
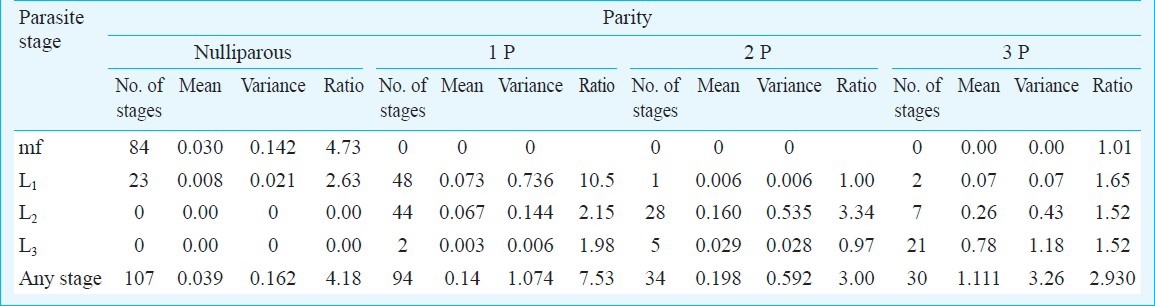
The mean and variance to mean ratio of infection intensity in the vector population in relation to parasite stage and vector age are presented in Table II. The overall intensity of parasites increases with vector age. However, aggregation of parasites, as indicated by variance to mean ratio, ranged from 4.1 in nulliparous to 7.5 in those mosquitoes that had single contact with the host (Table II). Since variance to mean ratio showed a decreasing trend in the mosquitoes that had two and more contacts with the host, density dependent parasite mortality is likely to be more in the older mosquitoes. Decreasing trend of variance to mean ratio for parasite stage L1 in the mosquitoes that had 2 and 3 contacts with the host indicated that parasite mortality for L1 stage was more in the older mosquitoes. Similarly, there was a clear evidence of parasite mortality for L2 and L3 among the mosquitoes that had 3 contacts with the host. The parasite distributions became less aggregated with larval stage in those mosquitoes which had only one contact with the host. Since the number of infected mosquitoes was less than 3 per cent of total mosquitoes dissected (N=3625), fitting of aggregated distribution viz., negative binomial was not possible to further corroborate the findings.
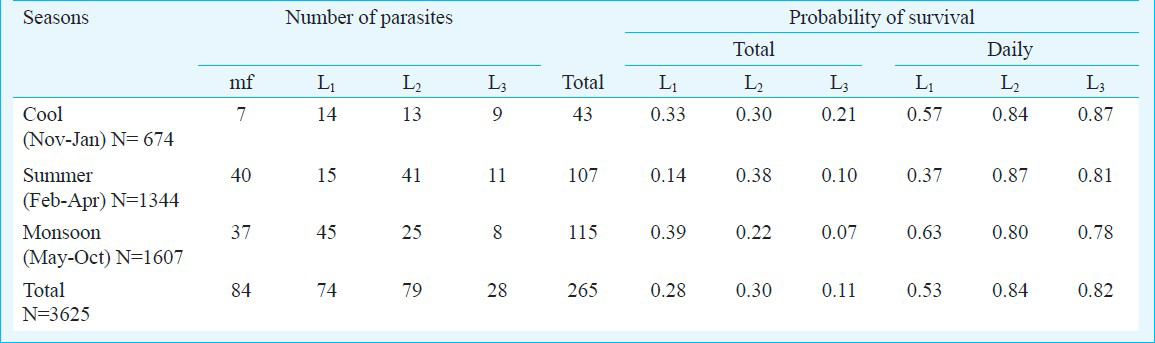
Survival probabilities of the parasite: Number of parasites in vector mosquito in relation to seasons is shown in Table III. The overall daily probability of survival for parasite stage L2 and L3 (>0.8) was markedly higher than that of L1 (0.53) stage. While the daily probability of survival for parasite stage L1 was noticeably less in summer season compared to the other two seasons, there was no evidence of seasonal influence on the probability of survival for later stages of parasites (L2 & L3). Since the findings were based on wild caught mosquitoes, it is likely that a proportion of mosquitoes might have acquired multiple infections. Therefore, it could not be ruled out that the daily probability of survival of parasites was from same cohort.
Discussion
Our study addresses an important question on the understanding of the factors that regulate parasite numbers in the vector and how such processes affect transmission of filariasis. There is a lacuna in the literature on the evidence for density dependent mortality of parasites or vectors. It was observed that the prevalence and intensity of infection declined with increasing stage of the parasite but increased with age of the vector. Similar observations have been reported for the W. bancrofti - Cx. quinquefasciatus combination1415. Since the parasite cannot either multiply or be transferred from one mosquito to another, the increase in both prevalence and intensity could be attributed to the accumulation of infection as the vector age increases. The reduction in prevalence of infection with increasing age of the parasite could either be due to density dependent parasite loss or mosquito mortality or to a combination of both factors. However, owing to complex host-parasite interactions it is difficult to estimate the survival rate of the mosquitoes based on parity alone14.
Further, the assessment of the degree of parasite aggregation in the vector population indicates that it falls markedly with parasite stage, as substantiated from the variance to mean ratio. Distributions of L3 are markedly less aggregated than earlier larval distributions as reported elsewhere1519. This suggests the function of density dependent parasite mortality and over dispersion of mf in human host could influence mf uptake by the mosquito while feeding, resulting in similar distribution pattern of mf in mosquitoes. However, the later stages of the parasite showed random distribution phenomenon in the vector, indicating the operation of density dependent phenomenon during the development of parasites in the vector. The distribution patterns of various filarial larval stages further suggested that loss of parasites occurred as development progressed and was maximal between the first and second larval stages.
Inconsistent observations have been reported on vector mortality due to parasite density. It has been observed that the infection of mosquitoes with parasites do not cause recognizable mortality25. However, it has been suggested that heavy infection of the vector mosquitoes can cause mortality which increases when the larvae reach the infective stage26. In the present study the variance to mean ratios indicated that parasite distributions became less overdispersed with parasite stage, particularly in the older stages (L2 & L3). As a result a lower proportion of vectors had a high parasite load of advanced developmental stages. This is considered as an indication of adverse effect of parasite infection on the vector host. The gap in the daily survival of vector was marked between L1 and L2 as observed in Cx. quinquefasciatus15 which suggested that the loss of parasite due to vector mortality was more during the development from L1 to L2.
The dynamics of infection in the vector mosquito are intricate; because both the acquisition and loss of infection are continuous processes as the vector can lose or gain infection during a subsequent blood meal. The relationship is further complicated by the different rates of survival of parasite and mosquito. The successful development of mf into infective larvae (parasite yield) is an essential component of successful transmission of the parasite. Consequently the transmission threshold for making decisions based on antigen/antibody prevalence needs to be verified for different parasite-vector combinations of filariasis. The revised guidelines for transmission assessment survey27 allow certain antigen positives in children. However, critical levels suggested for Culex and Aedes are lower than Anopheles transmitted filariasis keeping in view the limitation phenomenon28. The present study with Aedes transmitted filariasis conducted before launching LF elimination programme in the study area, indicates a comparable level of parasite regulation in the vector which has similar implications on the transmission threshold. Thus, the consideration of Aedes with Culex in deriving the critical level of antigen positive for making decisions on stopping mass drug administration (MDA) can be justified. However, with MDA aiming at reducing parasite load in the community, the operation of density dependent factor in the transmission becomes less pronounced in the subsequent rounds of MDA.
Acknowledgment
Authors thank Dr Shankar Saha, Medical Officer, Primary Health Centre, Teressa and the Director of Health Services, Andaman & Nicobar Administration, Port Blair, for extending their co-operation during the conduct of this study, and Drs K.D. Ramaiah and S. Subramanian, Vector Control Research Centre, Puducherry, for valuable suggestions and comments. The financial support from the Indian Council of Medical Research, New Delhi (No. 5/8-7(137) M98-ECD-II) is acknowledged.
References
- The economic burden of lymphatic filariasis in India. Parasitol Today. 2000;16:251-3.
- [Google Scholar]
- Medical organization and diseases of Andaman and Nicobar Islands. Abstr Trop Dis Bull. 1942;41:703-8.
- [Google Scholar]
- A note on malaria and filariasis in Andaman and Nicobar. Bull Natl Soc India Mal Other Mosq Dis. 1958;6:193-206.
- [Google Scholar]
- Filariasis among the aborigines of Andaman and Nicobar Islands. J Commun Dis. 1974;6:40-56.
- [Google Scholar]
- Phylogeny and classification of Aedini (Diptera: Culicidae), based on morphological characters of all life stages. Zool J Linn Soc. 2004;142:289-368.
- [Google Scholar]
- Filariasis in Andaman and Nicobar Islands. I. Survey findings-Nancowry, Teressa, Chowra, Car Nicobar and Port Blair. J Commun Dis. 1975;7:15-30.
- [Google Scholar]
- Epidemiology of sub- periodic Wuchereria bancrofti infection in the Nicobar Islands, India. Trans R Soc Trop Med Hyg. 1995;89:163-6.
- [Google Scholar]
- Prevalence of diurnally subperiodic bancroftian filariasis among the Nicobarese in Andaman and Nicobar Islands, India: effect of age and gender. Trop Med Int Health. 2002;7:949-54.
- [Google Scholar]
- Diurnal pattern of human-biting activity and transmission of subperiodic Wuchereria bancrofti (Filariidae: Dipetalonematidae) by Ochlerotatus niveus (Diptera: Culicidae) on the Andaman and Nicobar Islands of India. Am J Trop Med Hyg. 2005;72:273-7.
- [Google Scholar]
- Transmission dynamics of diurnally subperiodic lymphatic filariasis transmitted by Ochlerotatus (Finlaya) niveus in the Andaman & Nicobar Islands. Indian J Med Res. 2008;127:37-43.
- [Google Scholar]
- Population dynamics, age composition and survival of Downsiomyia nivea in relation to transmission of diurnally sub periodic filariasis. J Asia Pac Entomol. 2011;14:34-40.
- [Google Scholar]
- Vector transmission heterogeneity and the population dynamics and control of lymphatic filariasis. Adv Exp Med Biol. 2010;673:13-31.
- [Google Scholar]
- Ecological meta-analysis of density-dependent processes in the transmission of lymphatic filariasis: survival of infected vectors. J Med Entomol. 2009;46:873-80.
- [Google Scholar]
- Rates of acquisition and loss of Wuchereria bancrofti infection in Culex quinquefasciatus. Am J Trop Med Hyg. 1994;51:244-9.
- [Google Scholar]
- Frequency distribution of Wuchereria bancrofti infection in the vector host in relation to human host: evidence for density dependence. Acta Trop. 1995;60:159-65.
- [Google Scholar]
- Experimental infection of Anopheles gambiae and Culex pipiens fatigans with Wuchereria bancrofti in coastal East Africa. J Med Entomol. 1973;10:189-93.
- [Google Scholar]
- The relationship between microfilarial load in the human host and uptake and development of Wuchereria bancrofti microfilariae by Culex quinquefasciatus: a study under natural conditions. Parasitology. 1998;116:243-55.
- [Google Scholar]
- Processes influencing the distribution of parasite numbers within host populations with special emphasis on parasite-induced host mortalities. Parasitology. 1982;85:373-98.
- [Google Scholar]
- Loss of filarial larvae in a natural mosquito population. Ann Trop Med Parasitol. 1978;72:561-5.
- [Google Scholar]
- Observations on population trends of Culex quinquefasciatus Say, 1823 (Diptera: Culicidae) and transmission indices of bancroftian filariasis during and after integrated vector control programme in Pondicherry. In: PhD. Thesis. Puducherry, India: University of Pondicherry; 1990.
- [Google Scholar]
- Experimental studies on infection dynamics: infection of the definitive host by the cercariae of Transversotrema patialense. Parasitology. 1978;77:189-200.
- [Google Scholar]
- A new Aedes (Finlaya) of the Niveus-subgroup (Diptera: Culicidae) Mosq Syst. 1987;19:212-36.
- [Google Scholar]
- Determination of the physiological age of female Anopheles by the number of gonotropic cycle completed, Mediteinskaia Paraziologgia I. Parazitarnye Bolezini (Moskva). 1949;18:352-5.
- [Google Scholar]
- Human filariasis: a global survey of epidemiology and control. Tokyo: University of Tokyo Press; 1976.
- [Google Scholar]
- Some observations on filariasis in Western Samoa after mass administration of diethylcarbamazine. Trans R Soc Trop Med Hyg. 1976;70:39-48.
- [Google Scholar]
- On the escape of infective filarial larvae from the mosquitoes. Tropen Med Parasitol. 1977;28:461-6.
- [Google Scholar]
- WHO 2011. Monitoring and epidemiological assessment of mass drug administration in the global programme to eliminate lymphatic filariasis. A manual for national elimination programmes. Geneva: World Health Organization; 2011. p. :78.
- [Google Scholar]
- Factors affecting transmission of Wuchereria brancrofti by anopheline mosquitoes. 4. Facilitation, limitation, proportionality and their epidemiological significance. Trans R Soc Trop Med Hyg. 1992;86:523-30.
- [Google Scholar]






