Translate this page into:
Efficacy & safety of continuous erythropoietin receptor activator (CERA) in treating renal anaemia in diabetic patients with chronic kidney disease not on dialysis
Reprint requests: Dr Anil Bhansali, Professor & Head, Department of Endocrinology, Postgraduate Institute of Medical Education and Research, Chandigarh 160 012, India e-mail: anilbhansaliendocrine@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Chronic kidney disease (CKD) patients on dialysis regularly receive erythropoiesis stimulating agent (ESA) for treating renal anaemia during their dialysis unlike those who are not on dialysis. In such patients, the longer acting ESA can be helpful in reducing their frequent visits to the health care facilities and improving their compliance. This study was aimed to examine the efficacy and safety of continuous erythropoietin receptor activator (CERA), a long acting ESA in treating renal anaemia in patients with diabetic CKD not on dialysis.
Methods:
In this prospective, open-labelled, pilot clinical study, 35 adult type 2 diabetes patients with nephropathy and renal anaemia, who were not on dialysis nor receiving treatment with ESA were administered CERA subcutaneously once in two weeks for a period of 24 weeks. The primary efficacy end point was to evaluate the Hb response (Hb rise of ≥1 g/dl above the baseline or Hb level ≥11 g/dl) during the study period.
Results:
All patients showed Hb rise ≥1 g/dl during the study period and 80 per cent patients could achieve Hb value ≥11 g/dl. The maximum median Hb rise of 1.2 g/dl occurred in the initial 6 weeks after starting the treatment. The mean creatinine clearance (CrCl) improved by 2.8 ml/min, with mean Hb rise of 2.6 g/dl from the baseline after administration of CERA. Worsening of blood pressure (BP) control (42.9%) was the most common adverse event.
Interpretation & conclusions:
CERA once in two weeks was found to be efficacious in correcting anaemia in the ESA-naïve patients with diabetic nephropathy who are not on dialysis. However, regular monitoring of blood pressure is required while on treatment with CERA.
Keywords
Anaemia
continuous erythropoietin receptor activator
diabetic chronic kidney disease
type 2 diabetes mellitus
Anaemia is more common and severe in patients with diabetes than without diabetes, and the problem is further magnified in patients with renal impairment. The Third National Health and Nutrition Examination Survey (NHANES-III) showed that individuals with diabetes in the general population are nearly twice as likely to have anaemia compared to people without diabetes but with a similar degree of kidney function impairment1. In selected populations identified by screening programmes, such as the Kidney Early Evaluation Programme (KEEP), anaemia was detected in 30 per cent of diabetes patients with stage III chronic kidney disease (CKD), over twice the rate observed in patients without diabetes (14%)2. Similarly, clinic-based studies, such as the Prevalence of Anaemia in Early Renal Insufficiency (PAERI) study, have detected a higher prevalence of anaemia in patients with diabetes and CKD than in those with CKD but not diabetes (52.7 vs 39.4%; P <0.01)1.
Erythropoiesis-stimulating agents (ESA) have become the cornerstone of therapy to treat renal anaemia since these were introduced two decades ago34. Initial available ESA namely epoetin alpha and epoetin beta had short half life and were needed to be administered twice or thrice a week5. Continuous erythropoietin receptor activator (CERA) is a newer ESA with long half-life and with less frequent dosing of once every two week or even once monthly. CERA exhibits a long half-life of approximately 77 h6 and 142 h in CKD patients not on dialysis7 and 134 h and 139 h in CKD patients receiving dialysis8, when administered intravenously and subcutaneously, respectively. Patients on dialysis are usually supplemented with ESA for treating renal anaemia during their dialysis unlike those who are not on dialysis. Longer acting ESA may be helpful in reducing the frequency of injections and number of hospital visits resulting in better compliance. We undertook this study to examine the efficacy and safety of subcutaneous administration of CERA once in two week in treating renal anaemia in patients with diabetic nephropathy who are not on dialysis.
Material & Methods
This was a prospective, open-labelled, single-arm, non-comparative, pilot clinical study conducted during January to November 2010 in Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. Consecutive diabetic CKD patients [stage III and IV with crectinine clearance (CrCl) between 15 and 60 ml/min, calculated by modification of diet in renal disease (MDRD formula] with renal anaemia (Hb between 8 and 10 g/dl with serum ferritin >100 ng/ml) attending the Outpatients Department of Endocrinology and Nephrology Clinics of PGIMER, Chandigarh, India, received CERA administered subcutaneously once in two week using prefilled syringes for 24 week duration. The study protocol was approved by the institute's ethics committee and written informed consent was taken from the patients (Clinical Trial Registry-India, Trial No.2009000622).
Study design: After screening, the patients underwent a run-in period observed for four weeks during which patients continued to receive oral iron, folic acid and vitamin B12 supplementations along with their anti-diabetic and anti-hypertensive medications. Those patients having lower serum ferritin (<100 ng/ml) were administered 400 mg of parenteral iron and repeat serum ferritin was done at 0 week to ensure adequate iron reserve in the body before administering CERA. Ferritin levels ranged from 110 to 764 ng/ml with median value of 217.
The study consisted of first 12 wk (1-12 wk) of dosage titration and Hb correction period followed by 12 wk (13-24 wk) of efficacy evaluation period. After run-in period, patients received subcutaneous CERA, 0.6 μg/kg every 2 wk9.
During the correction period, the dosage of CERA was adjusted to achieve Hb level ≥ 11 g/dl. During the extension period, Hb levels were to be maintained between 11 and 12 g/dl. Dosage adjustments were performed according to a predefined protocol but no more frequently than once every four wk unless safety concerns dictated otherwise. The need for dosage adjustment was based on two consecutive Hb assessments. Upto achievement of response, CERA dosages were increased by 25 per cent for Hb increase <1 g/dl above baseline Hb in a 4-wk period. CERA dosages were decreased by 25 per cent for Hb increase >2 g/dl or Hb value >13 and ≤14 g/dl. If Hb >14 g/dl therapy was interrupted till Hb <13 g/dl and then restarted at 25 per cent reduced dose. During the study period, patients received daily oral iron (200 mg of elemental iron daily), folic acid 5mg and vitamin B12 (1000 μg). Serum ferritin was repeated at 12 wk and at the end of study at 24 wk and if <100 ng/ml was corrected by parenteral iron.
Assessment: Patients were scheduled for assessment once every two weeks during the study. Blood pressure monitoring and laboratory parameters such as Hb and serum creatinine were performed at each visit. The primary efficacy end point was to see Hb response which was defined as Hb value of ≥11 g/dl or Hb rise ≥1 g/dl from the baseline Hb value at any time during the study period. The secondary efficacy end points were to calculate the incidence of any adverse events during the study and the effect of mean Hb rise due to CERA in the creatinine clearance of diabetic CKD patients.
Statistical analysis: Student paired‘t’ test was used to find the significance of difference between the measured values and P < 0.05 was considered significant. The analysis was done for target-to-treat (TTT) group (all patients without major protocol violation).
Results
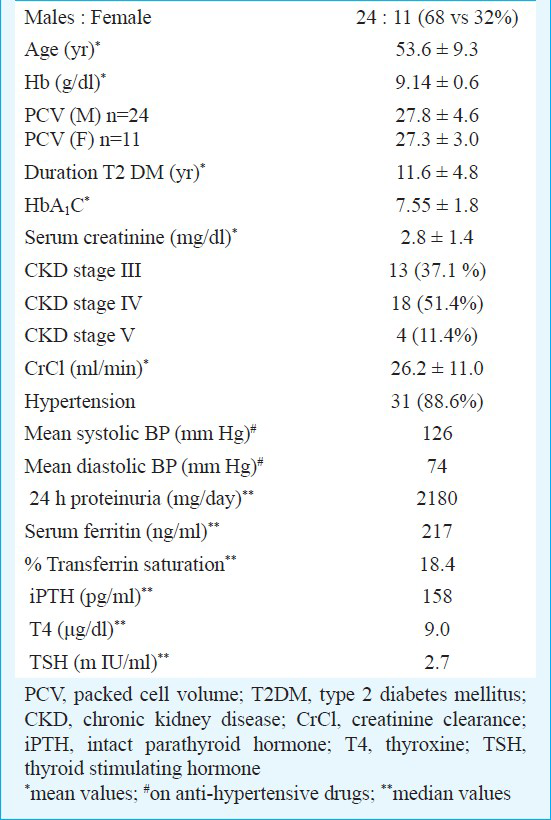
After screening 69 patients, 35 patients were enrolled in the study. Of these, 31 patients were included in intention-to-treat (ITT) group (88.6%). The reasons for withdrawal of the 4 patients were haemodialysis (n=1), refusal of treatment (n=1) and failure to return (n=2). Six patients were excluded from ITT group, to form TTT group, which comprised 25 patients (71.4 %). The main reasons for exclusion were haemodialysis (n=2), death (n=2) and failure to return (n=2). The baseline characteristics were as mentioned in the Table.
Efficacy evaluation: All patients in TTT group (n=25) had Hb rise ≥1 g/dl and 80 per cent patients (n=20) had Hb value ≥11 g/dl any time during the study period. The mean Hb rise of 2.58 g/dl from baseline (9.10 versus 11.68 g/dl, P≤0.001) was accompanied with an increase in the packed cell volume (PCV) value of 6.5 per cent (27.4 versus 33.9%, P≤0.001) and also improvement in CrCl value of 2.8 ml/min (26.2 ±11.0 versus 29.0±16.7, P=0.3), at the end of the study (Fig.).
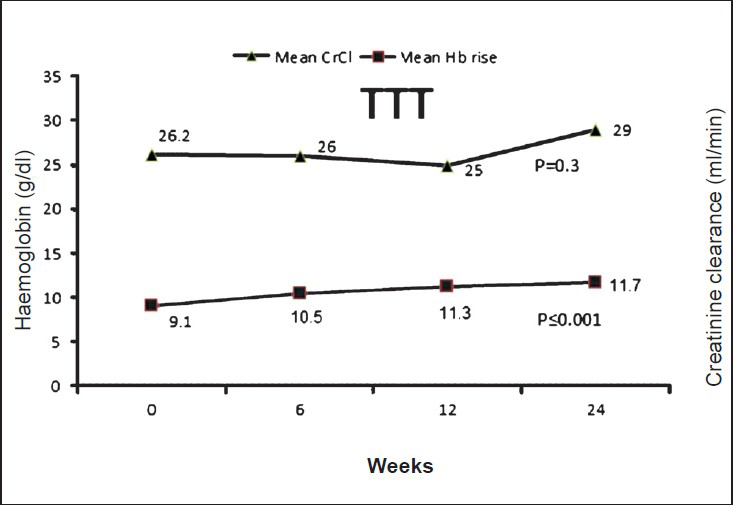
- Relationship between mean Hb rise and alteration in creatinine clearance (CrCl) due to CERA from baseline and at 24 wk.
Of the 25 patients in TTT group, five had their haemoglobin value reached beyond the value of 13 g/dl even on the minimum dose of 50 μg given at once in two wk and as per predetermined protocol it was required to reduce the dose by 25 per cent, however, the same dose was given at the interval of 4 wk as pre-filled syringe with less than 50 μg was not available.
Adverse events: The most commonly reported adverse event was worsening of BP which was seen among 42.9 per cent patients (n= 15). Worsening of BP was defined as systolic BP (SBP) >140 mm Hg and diastolic BP >90 mm Hg. The other commonly reported adverse events were worsening of renal functions and gastritis seen in 11.42 per cent patients (n=4) each, fatigability for 3-4 days following injection, diarrhoea and hypotension which were seen in 8.6 per cent patients (n=3) each. There were two deaths during the study period, unrelated to study medication. One was severe sepsis-related and another was due to acute coronary event in a patient with underlying cardiovascular co-morbidities.
Discussion
The present study showed that CERA was effective to treat renal anaemia in diabetic CKD patients, not on dialysis. However, regular monitoring of BP is required while on treatment with CERA.
Renal anaemia in diabetic CKD patients tends to occur earlier and is more severe than non-diabetic CKD. Majority of the studies available for treating renal anaemia are available on dialysis patients. In the Administration of CERA in Chronic kidney disease patients to treat anaemia with Twice –monthly Schedule (ARCTOS) trial, 97.5 per cent patients who received CERA once in every two weeks achieved the Hb level between 11-13 g/dl which was higher than in our study but the mean change in the Hb from baseline to evaluation period was 2.1 g/dl (10.2 versus 12.3 g/dl) which was similar to our results10.
Once the target is achieved, the drug can be used either at 0.6 μg/kg once in 2 wk or 1.2 μg/kg (i.e. double the dose) once in the 4 week during the maintenance phase. The present study was designed to continue the administration of drug at 2 wk interval even during the efficacy evaluation phase. In the five patients who received 0.6 μg /kg dose at interval of 4 wk instead of 2 wk the haemoglobin remained above the target value (>11 g/dl) which was also seen in the monthly injection realized continues erythropoietin receptor activator (MIRACEL)study in the haemodialysis patients11 and in ARCTOS extension study in non-dialysis patients10.
The most common adverse event (AE) during the study was worsening of hypertension, which was also seen in patients receiving CERA in ARCTOS trial10 and it is due to impairment of both the endothelium-dependent as well as endothelium-independent vasodilation12. In this study, not all known hypertensive patients had worsening of their BP but regularly BP monitoring is necessary for early detection of the rise in BP and timely controlling it in to avoid adverse cardiovascular complication and stroke. There was slight improvement in CrCl value but more studies would be required to evaluate this effect of CERA.
There were certain limitations in this study. Firstly, the study was for a short duration and the sample size was small. Secondly, it was an open-labelled study and so may have its impact on the data, particularly on the incidence and severity of any AE. Thirdly, the improvement in the quality of life (QoL) score was not assessed and also the effect of the treatment on the major and the minor chronic complications of diabetes mellitus was also not assessed. Also, the erythropoietin antibody testing was not performed during the study.
To conclude, this study demonstrated that CERA administered subcutaneously once every two wk was effective in increasing the Hb as well as maintaining target Hb in the diabetic CKD patients not on dialysis. However, regular monitoring of blood pressure is required while on treatment with CERA.
References
- Association of kidney function with anemia: the Third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2002;162:1401-8.
- [Google Scholar]
- Kidney Early Evaluation Program 2004 KEEP 2004 Data Report. Am J Kidney Dis. 2005;45(Suppl 2):S8-S13.
- [Google Scholar]
- Effect of human erythropoietin derived from recombinant DNA on the anaemia of patients maintained by chronic haemodialysis. Lancet. 1986;2:1175-8.
- [Google Scholar]
- Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N Engl J Med. 1987;316:73-8.
- [Google Scholar]
- Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clin Pharmacol Ther. 1991;50:702-12.
- [Google Scholar]
- Methoxy polyethylene glycol-epoetin beta: a review of its use in the management of anaemia associated with chronic kidney disease. Drugs. 2008;68:1139-56.
- [Google Scholar]
- MIRCERA (methoxy polyethylene glycol-epoetin beta) in the tretment of patient with chronic kidney disease presenting late-onset hypersensitivity ot other epoetins. Nefrologia. 2010;30:372-3.
- [Google Scholar]
- C.E.R.A: pharmacodynamics, pharmacokinetics and efficacy in patients with chronic kidney disease. Expert Opin Investig Drugs. 2007;16:1649-61.
- [Google Scholar]
- The continuous erythropoietin receptor activator (C.E.R.A) corrects anemia at extended administration intervals in patients with chronic kidney disease not on dialysis: results of a phase II study. Clin Nephrol. 2007;67:306-17.
- [Google Scholar]
- C.E.R.A corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial. Clin J Am Soc Nephrol. 2008;3:337-47.
- [Google Scholar]
- Evaluation of maintenance of stable haemoglobin levels in haemodialysis patients converting from epoetin or darbepoetin to monthly intravenous C.E.R.A: the MIRACEL study. Curr Med Res Opin. 2010;26:1083-9.
- [Google Scholar]
- Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA) Clin J Am Soc Nephrol. 2009;4:470-80.
- [Google Scholar]






