Translate this page into:
Entomological investigations into an epidemic of Japanese encephalitis (JE) in northern districts of West Bengal, India (2011-2012)
Reprint requests: Dr B.K. Tyagi, Centre for Research in Medical Entomology (ICMR) #4. Sarojini Street, Chinna Chokkikulam, Madurai 625 002, India e-mail: crmeicmr@icmr.org.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Japanese encephalitis (JE) is one of the most important arboviral diseases of human beings with outbreaks in many parts of Southeast Asia including India. We present the entomological findings of an outbreak occurred in northern part of West Bengal during 2011-2012 with special emphasis on the role of JE vectors in different seasons.
Methods:
Adult mosquito collections were made with the help of mouth aspirators, aided by flash lights during day time resting inside human and animal habitations as indoor, and resting outside field grasses, bushes, underneath of culverts and bridges as outdoor, and in and around the pig enclosures and cattle sheds during dusk period in JE affected villages from Cooch Behar, Dakshin Dinajpur, Darjeeling and Jalpaiguri districts in North West Bengal. In all study villages, a long handled with enamel bowl dipper was used to obtain immature stages of mosquitoes from various breeding habitats.
Results:
A total of 19 different types of mosquito breeding habitats were examined for vectors of JE. From these habitats, 23.7 per cent were positive for breeding during the study period. Overall, nine different species were recorded through emergence, but none was positive for JE virus when subjected for detection of virus. Adult mosquitoes of more than 50 per cent of the potential JE vector species obtained through dusk and the rest through indoor and outdoor collections in all seasons. Altogether, 27 different species were recorded. Most of these were JE vectors.
Interpretation & conclusions:
Our results showed that in addition to Cx. vishnui subgroup, detection of JE virus antigen in Cx. quinquefasciatus indicated the possible maintenance of JE virus in nature through poor vector mosquitoes throughout the year. Since, all potential vector species reported elsewhere in India were also found in this region and fluctuated in density in different seasons, a proper integrated vector control programme needs to be implemented to control JE transmission.
Keywords
Culex pseudovishnui
Cx. quinquefasciatus
C. tritaeniorhynchus
Cx. vishnui
Japanese encephalitis
mosquito emergence
vector
Japanese encephalitis (JE) is a mosquito-borne arboviral disease and is the leading cause of morbidity and mortality in South East Asia. The outcome of JE can be fatal in 25 per cent of cases, and it may also result in residual sequelae in 30 to 60 per cent of cases12. In India, the disease was first reported in 1955 and subsequently many epidemics have occurred in different parts of the country34567. JE virus (JEV) infects a large number of susceptible individuals but only a few develop overt manifestations of the disease8. JE is principally a disease of rural agricultural areas, where vector mosquitoes proliferate in close association with pigs, which are the principal vertebrate amplifying hosts. Large aquatic birds may sub serve this function in areas where pigs are absent. Humans are incidental to the transmission cycle910. The seasonal incidence of JE varies in different parts of India. In West Bengal, the disease occurred between May and October, and was shown to be related to the summer monsoon. In Assam and Uttar Pradesh epidemics have occurred between September and December11. During 2011-2012, JE outbreaks occurred in many parts of north eastern States including Bihar and North West Bengal. During the recent outbreak JE cases were reported from four different districts (i.e. Cooch Behar, Dakshin Dinajpur, Darjeeling and Jalpaiguri) of North West Bengal from the first quarter of 2011 onwards. After confirmation of JE infection, an entomological investigation was initiated by the team of Centre for Research in Medical Entomology (CRME), Madurai, Tamil Nadu, India, in September 2011 onwards to complete a year round study at the quarterly interval to pin point the role of different species of mosquito vector. The results of this entomological investigation are presented here.
Material & Methods
Study area: After the JE cases reported in the four districts of Northern West Bengal, four surveys were carried out at the quarterly intervals (i.e. September 15, 2011 to October 2, 2011 in 19 villages, January 18, 2012 to February 6, 2012 in 19 villages, May 10, 2012 to June 3, 2012 in 24 villages and September 11, 2012 to October 10, 2012 in 24 villages) in the four districts.
As per the climatological particulars provided by the Government of West Bengal Office of the Additional Director of Agriculture, North Bengal Region, Jalpaiguri, the JE affected four different regions have five distinct seasons (summer - April to May, monsoons - June to September & October to November, autumn - December to January middle, winter - January middle to February middle, and spring - February to March). The average rain fall (424.8 mm) occurs in June only. The relative humidity ranges between 60.5 per cent in March and 84.6 per cent in October. The annual mean maximum temperature is 14.9 °C while the mean minimum temperature is 8.9 °C with monthly mean temperatures ranging from 5-17 °C. The average annual precipitation is 309.2 cm, with an average of 126 days of rain in a year. The highest rainfall occurs in July.
Study design: The four districts made a single study unit. of the four districts, a total of 42 villages were selected (as per the line list submitted by the North West Bengal Medical College Hospitals, Department of Health, West Bengal). Of these 42 villages, four visits were made in nine villages, three visits in seven, two in eight and 13 single visits and the remaining five were not attended due to logistic reasons. Overall, 86 collections of immature stages from various breeding habitats and different types of adult mosquitoes collections from these 37 villages were made.
The criteria followed included minimum three villages from respective districts to study at the quarterly interval. However, nine villages were covered from three districts as designed only, three villages could not be studied from Dakshin Dinajpur during the second visit thereby only three visits made in those three villages (i.e. first, third and fourth visits). The remaining villages were covered either single or 2 or 3 visits only as stated above. Most of the sites were under paddy cultivation plus other geographical variations with different altitudes (ranged between 66 and 1500 feet) located behind riverside, tea garden cultivation, adjacent to forested, elevated hilly tracts, very close to big water stored ponds, etc. These study sites were chosen on the basis of line list of JE confirmed cases.
Adult mosquitoes collection: In every study village, the various types of mosquito collections undertaken included indoor, outdoor and dusk for adult mosquitoes at the quarterly interval from September 2011 to October 2012. In respective study sites, during day time (i.e. 0900 and 1000 h) mosquito catch was made from inside houses including sitting room, bed room, store room and kitchen with the help of mouth aspirators aided by torch lights. Similar collections of resting mosquitoes made from outdoor resting habitats consisted of field grasses, bushes, fencing of open area, underneath of culverts, etc. Dusk mosquito collections (from 1915 to 2015 and 1750 to 1850 h during summer and winter, respectively) were made with the help of mouth aspirators, aided by torch lights in and around pig enclosures and cattle sheds. In identified study villages, per man hour density (PMH) was monitored for each and every types of collections known as indoor, outdoor and dusk. Both blood engorged and unfed adult mosquitoes were subjected for virus isolation12131415.
Collections of immature stages: Study villages, various types of breeding habitats were examined for mosquito immature stages by spending not less than an hour with dipper (i.e. an enamel bowl with 12.5 cm in diameter fitted with one meter long handle of aluminium rod). The required dips were obtained depending upon the size of various habitats like pits, pools and paddy fields, etc. In drain or canal sequential sampling was done at the gap of 2 meters on either side14. Immature stages of late fourth instar and pupae obtained were kept for emergence to identify the species.
Screening mosquitoes for JE virus: Mosquitoes obtained by different methods were identified upto species level and made into pools in the transit laboratory. The pools were kept in liquid nitrogen and brought to the central laboratory, CRME, Madurai, to examine for virus detection by antigen capture ELISA method employing JE virus specific monoclonal antibody15.
Statistical analysis: In the present study, approximately eight per cent houses were selected as sample from the sample frame. The appropriate statistical measures were applied along with graphical presentation representing seasonal comparison between various genera of mosquitoes. Per man hour density of mosquitoes were calculated for adult mosquitoes collections.
Results
Immature stages of mosquitoes: In all study sites, a total of 19 different types of mosquito breeding habitats were examined for breeding status (Table I). Of these 19 habitats, 23.7 per cent were found positive for breeding of mosquitoes. The positive breeding habitats in September-October 2011 were 23.2 per cent, 19.8 per cent in January-February 2012, 27.3 per cent in May-June 2012 and 24.9 per cent in September-October 2012. All emerged mosquitoes included Culex quinquefasciatus, Cx. tritaeniorhynchus, Cx. gelidus, Cx. vishnui, Cx. bitaeniorhynchus, Cx. fuscocephalus, Anopheles peditaeniatus, An. barbirostris, and Armigeres subalbatus. All emerged mosquitoes were subjected for virus isolation, but none of these emerged mosquitoes were positive for virus in the laboratory.
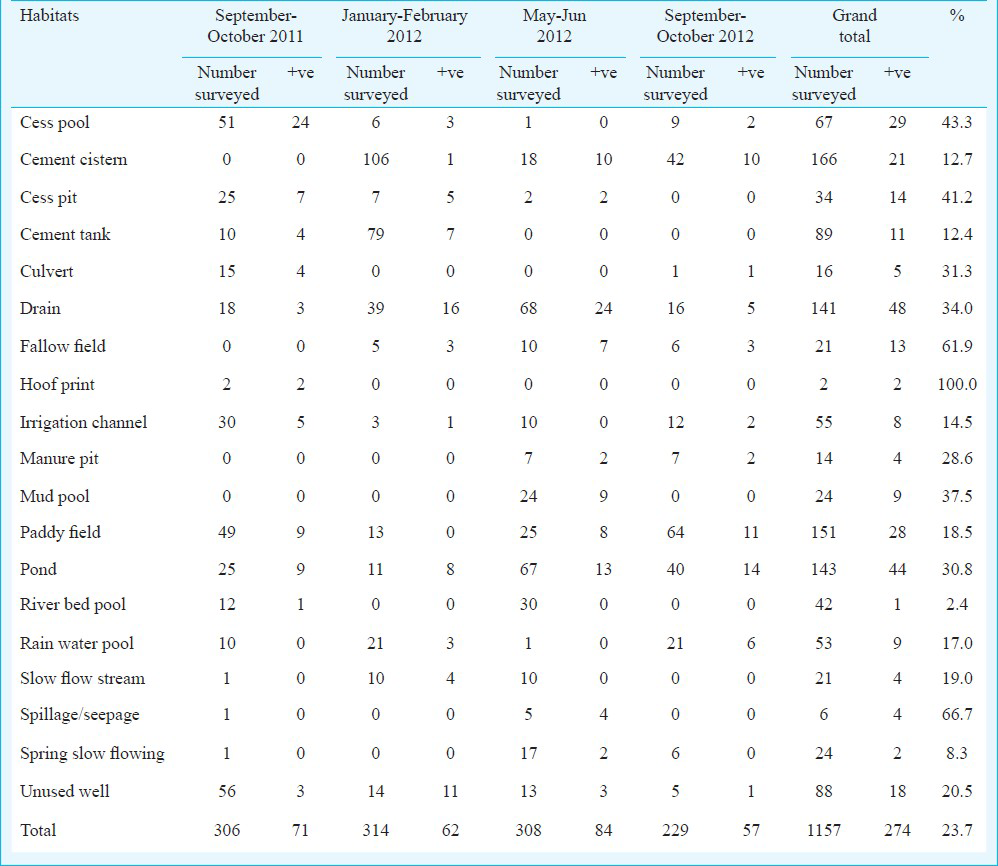
Adult mosquito collections: The adult mosquitoes obtained through various collections are shown in Table II. Indoor resting mosquitoes comprised 20 species belonging to six genera. Culex was the predominant genera followed by Armigeres, Anopheles, Mansonia, Aedes and Metalutzia.
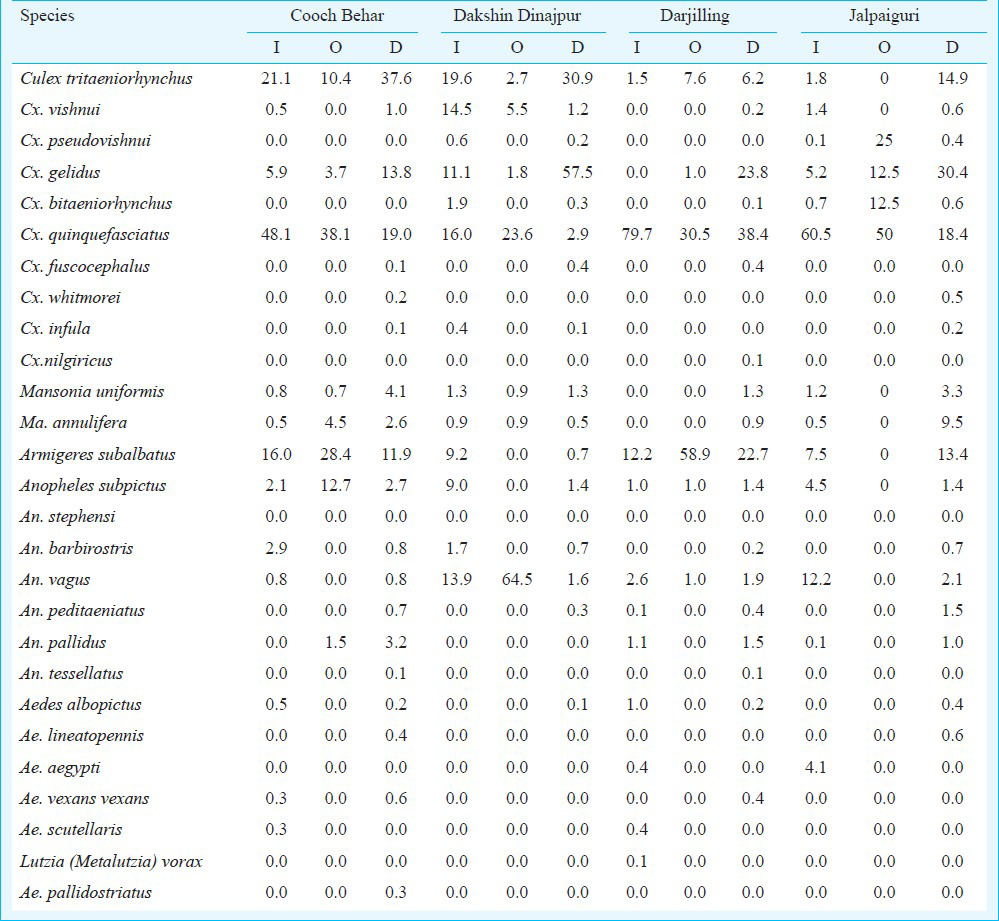
Among Culex mosquitoes, Cx. quinquefasciatus (57.54%) was predominant followed by Cx. tritaeniorhynchus (7.88%), Cx. gelidus (4.46%), Cx. vishnui (3.18%), Cx. bitaeniorhynchus (0.56%) and Cx. pseudovishnui (0.16%) and Cx. infula (0.08). of the five Anopheles species, An. vagus (7.2%) was predominant followed by An. subpictus (5.25%), An. barbirostris (0.76%), An. pallidus (0.44%) and An. peditaeniatus (0.08%). Within two Mansonia species, Ma. uniformis (1.63%) and Ma. annulifera (0.4%) were recorded. The remaining six different species of non-JE vectors namely Ar. subalbatus (9.27%), Ae. aegypti (1.35%), Lutzia (Metalutzia) vorax (0.08%), Ae. scutellaris (0.2%), Ae. albopictus (0.44%) and Ae. vexans vexans (0.08%) were recorded. In all study areas, Cx. quinquefasciatus obtained from indoor in all seasons were compared with conventional JE vectors (Fig.). The percentages of Cx. quinquefasciatus density obtained in descending order were 95.6 per cent in January-February 2012 (cold months), 83.4 per cent in May-June 2012 (summer months) and 44. 3 per cent in September-October 2011 (rainy months) and 10.4 per cent in September-October 2012.
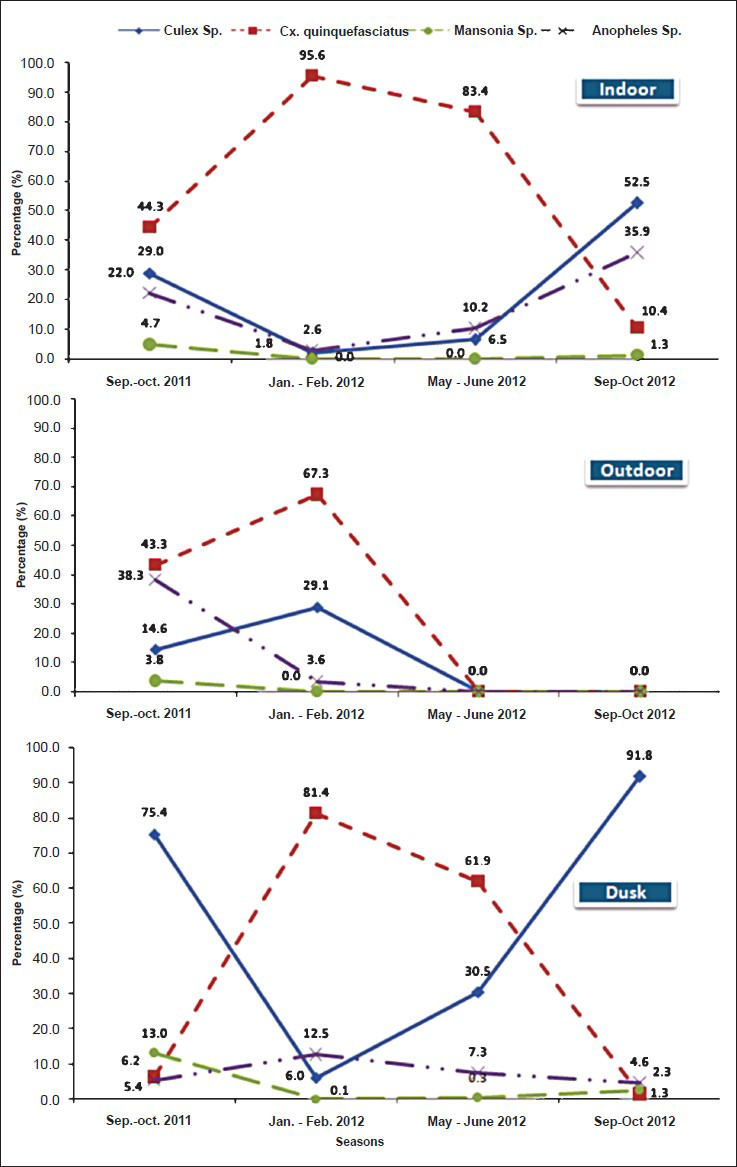
- Conventional JE vectors compared with Cx. quinquefaciatus obtained through indoor, outdoor and dusk collections
Outdoor resting mosquitoes obtained from the study areas comprised 12 species belonging to four genera. Culex was predominant genera followed by Armigeres, Anopheles, and Mansonia. Among Culex mosquitoes Cx. quinquefasciatus (35.55%) was predominant followed by Cx. pseudovishnui (6.25%), Cx. tritaeniorhynchus (5.18%), Cx. gelidus (4.75%), Cx. bitaeniorhynchus (3.13%) and Cx. vishnui (1.38%). Anopheles vagus (16.38%) was predominant followed by An. subpictus (3.43%) and An. pallidus (0.38%). In the case of Mansonia, Ma. annulifera (1.35%) and Ma. uniformis (0.4%) were obtained. The remaining non-JE vector Ar. subalbatus was also recorded (21.83%). Both indoor as well as outdoor obtained conventional vectors of all Culex species were compared respectively as shown in the Figure. The percentage of Cx. quinquefasciatus density obtained was 67.3 per cent in January-February 2012 (cold months) 43. 3 per cent in September-October 2011 (rainy months), and the collection was not done in the remaining two seasons. In the case of conventional Anopheles species, the peak was during rainy months followed by cold period and Mansonia species was recorded in very low numbers compared with other conventional vector mosquitoes.
Based on dusk collection, a total of 24 different mosquito species were obtained. Those species include 10 Culex (i.e. 80.39%) which was predominant followed by seven Anopheles (5.42%), two Mansonia (4.75%), four Aedes (0.54%), and one Armigeres (8.9%) (Table II). Among Culex mosquitoes, Cx. gelidus (39.36%) was predominant followed by Cx. tritaeniorhynchus (24.32%), Cx. quinquefasciatus (14.87%) Cx. vishnui (0.86%), Cx. bitaeniorhynchus (0.29%), Cx. fuscocephalus (0.29%), Cx. pseudovishnui (0.15%), Cx. whitmorei (0.13%), Cx. infula (0.11%) and Cx. nilgiricus (0.01). Of the seven Anopheles species, An. vagus (1.62%) was predominant followed by An. subpictus (1.6%), An. pallidus (0.96%), An. barbirostris (0.62%), An. peditaeniatus (0.58%), An. tessellatus (0.03%). and An. stephensi (0.01%). Among Mansonia species, Ma. annulifera was 2.62% followed by Ma. uniformis (2.13%) The remaining species included Ar. subalbatus (8.9%), Ae. vexans vexans (0.18%), Ae. lineatopennis (0.17%), Ae. albopictus (0.15%) and Ae. pallidostriatus (0.05%). In dusk collection, the percentage of Cx. quinquefasciatus density was 81.4 per cent in January-February 2012 (cold months) 61.9 per cent in May-June 2012, 6.2 per cent in September-October 2011 (rainy months), and 1.3 per cent in September-October 2012 (rainy months).
The percentage of Cx. quinquefasciatus contribution was low compared with conventional Culex mosquitoes obtained during rainy months where more habitats supported conventional Culex species. Other than Cx. quinquefasciatus, two peaks were noticed by the contribution of major vectors of JE, Cx. tritaeniorhynchus followed by Cx. vishnui, Cx. bitaeniorhynchus, Cx. fuscocephalus, Cx. pseudovishnui, Cx. whitmorei, Cx. infula and Cx. nilgiricus. Per man hour (PMH) densities of mosquitoes in the four different periods covering all four districts resulted from September-October 2011 to September-October 2012 were 85.13, 19.77, 34.45 and 129.25 PMH. The highest density recorded in rainy months indicated very high risk of transmission of JE during that particular period.
Detection of JE virus in mosquitoes: All mosquitoes were subjected to viral assay and found positive for JE virus which in a descending order with positive pools in parentheses were as follows: Cx. tritaeniorhynchus (3), Cx. gelidus (3), Cx. quinquefasciatus (2), Cx. pseudovishnui (2) Ma. uniformis (2), Cx. vishnui (1) Cx. fuscocephalus (1) Ma. annulifera (1). A total of 15 pools were found positive for JE virus antigen during the entire study period from eight different species. Most of the pools found positive for JE virus in rainy months proved the active virus circulation in this area and indicated a high risk of transmission due to the major vectors in this region.
Discussion
In the four districts surveyed in North West Bengal, the adult mosquitoes collections indicated that the various habitats were contributing vectors throughout the year. More than 50 per cent of the potential JE vector species obtained through dusk collection and the remaining through other collections pointed out that people in this area were at a risk for transmission of JE. Most of the species were potential vectors and very few were non-vector mosquitoes representing genera Anopheles, Aedes and Armigeres. Therefore, the area was prone for outbreak situation of JE in different seasons with a relatively high risk during rainy months.
JE virus is transmitted naturally between ardeid birds and pigs by mosquito species belonging to the Cx. vishnui subgroup, comprising Cx. tritaeniorhynchus, Cx. vishnui and Cx. pseudovishnui in India16. The present epidemic in the districts of North West Bengal confirmed the role of Cx. vishnui subgroup in virus transmission. The significant detection of JE virus antigen in Cx. quinquefasciatus indicated the possible maintenance of JE virus in nature through poor vector mosquitoes throughout the year. Systematic studies are needed to prove the role of Cx. quinquefasciatus in maintenance/transmission of JE virus with molecular tools. It has been reported that Cx. quinquefasciatus is the most common domestic species in urban, semi urban and rural areas and strongly anthropophagic, and JE virus isolation has been made from Kolar district, Karnataka171819. Though JE is a vaccine preventable disease20, the transmission can be reduced drastically by exercising vector control measures in JE endemic areas. In paddy fields, composite fish culture for controlling mosquitoes is being practiced in many parts of our country21 needs to be exercised in this region also. In Bellary district of Karnataka Cx. tritaeniorhynchus was found to be resting indoors in large numbers during JE transmission and house spray was suggested with residual insecticide to suppress the adult population22. However, in the JE affected belt in North West Bengal, the densities of the Culex subgenera species were found throughout the year increasing the chances of transmission. Since epidemics of JE continue to occur in different parts of the country and the diseases is spreading to newer areas, in-depth intensive studies need to be undertaken to highlight the role of different vector species towards JE transmission and to devise appropriate feasible control measures.
Acknowledgment
The authors acknowledge Dr V. M. Katoch, the Secretary, Department of Health Research and Director-General of Indian Council of Medical Research, for his support and encouragement; various North West Bengal Health staff for rendering their assistance in the field; and all the laboratory and field Staff of Centre for Research in Medical Entomology (CRME) for their assistance in carrying out the studies. We thank Shriyut R. Krishnamoorthi, K. J. Dhananjeyan, J. Nagaraj and A. Venkatesh for facilitating in illustration work.
References
- Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89:766-74.
- [Google Scholar]
- Epidemiology and surveillance of Japanese encephalitis in Tamil Nadu. ICMR Bull. 1998;28:33-7.
- [Google Scholar]
- A long-term study on vector abundance & seasonal prevalence in relation to the occurrence of Japanese encephalitis in Gorakhpur district, Uttar Pradesh. Indian J Med Res. 2003;117:104-10.
- [Google Scholar]
- The effects of climatic factors on the distribution and abundance of Japanese encephalitis vectors in Kurnool district of Andhra Pradesh, India. J Vector Borne Dis. 2010;47:26-32.
- [Google Scholar]
- Isolation of Japanese encephalitis virus from mosquitoes collected in Karnataka state, India from 1985 to 1987. Trans R Soc Trop Med Hyg. 1989;83:550-2.
- [Google Scholar]
- Mosquito vectors of Japanese encephalitis epidemic (1983) in Mandya district (India) Indian J Med Res. 1984;80:377-89.
- [Google Scholar]
- Outbreak of encephalitis in Bellary district of Karnataka & adjoining areas of Andhra Pradesh. Indian J Med Res. 1990;91:328-30.
- [Google Scholar]
- The first epidemic of Japanese encephalitis studied in India-virological studied. Indian J Med Res. 1975;63:77-82.
- [Google Scholar]
- A note on the 1976 epidemic of Japanese encephalitis in Burdwan district, West Bengal. Indian J Med Res. 1978;68:3938.
- [Google Scholar]
- Ecological studies on the mosquito vectors of Japanese encephalitis. Bull World Health Organ. 1973;49:287-92.
- [Google Scholar]
- Studies on the mosquito vectors of Japanese encephalitis virus in Mandya district, Karnataka, India. Southeast Asian J Trop Med Public Health. 1994;25:378-82.
- [Google Scholar]
- Ecological studies on Culex tritaeniorhynchus as a vector of Japanese encephalitis Bull. World Health Organ. 1973;49:41-7.
- [Google Scholar]
- Comparative evaluation of bioassay and ELISA for detection of Japanese encephalitis virus in field collected mosquitoes. Southeast Asian J Trop Med Public Health. 1995;26:91-7.
- [Google Scholar]
- Mosquito blood feeding patterns as a factor in the epidemiology of Japanese encephalitis in southern India. Am J Trop Med Hyg. 1992;46:654-63.
- [Google Scholar]
- Experimental transmission of Japanese encephalitis virus through Anopheles tessellatus and Culex fatigans mosquitoes. Indian J Med Res. 1977;65:746-52.
- [Google Scholar]
- Isolation of Japanese encephalitis virus from mosquitoes collected in Karnataka state, India from 1985 to 1987. Trans R Soc Trop Med Hyg. 1989;83:550-2.
- [Google Scholar]
- Isolation of Japanese encephalitis virus from mosquitoes collected in Bankura district, West Bengal during October 1974 to December 1975. Indian J Med Res. 1979;69:201-5.
- [Google Scholar]
- Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet. 1998;351:1094-7.
- [Google Scholar]
- Composite fish culture for mosquito control in rice fields in southern India. Southeast Asian J Trop Med Public Health. 1994;25:522-7.
- [Google Scholar]
- Ecological study on mosquito vectors of Japanese encephalitis virus in Bellary district, Karnataka. Indian J Med Res. 2007;126:152-7.
- [Google Scholar]






