Translate this page into:
Toll-like receptors, cytokines & nitric oxide synthase in patients with otitis media with effusion
Reprint requests: Dr Seung Geun Yeo, #1 Hoegi-dong, dongdaemun-gu, Seoul 130-702, Korea e-mail: yeo2park@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Microbial infections in the normally sterile environment of the middle ear cavity in patients with otitis media trigger expression of Toll-like receptors (TLRs), cytokines, and nitric oxide. We evaluated the expression levels of TLR-1, -2, -4, -5, -6, and -9, interleukin (IL)-6, -8, -10, and -12, interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), and nitric oxide (NO), in paediatric patients with otitis media with effusion (OME).
Methods:
The levels of TLR, cytokine, and nitric oxide synthase (NOS) mRNAs in middle ear effusion were assessed by real-time polymerase chain reaction in 96 children with OME, 24 prone and 72 not prone to otitis. The level of expression of each mRNA was compared in the otitis-prone and non-otitis-prone groups, in patients with and without bacteria, and by frequency of ventilation tube insertion.
Results:
The expression of TLR-1, -2, -4, -5, -6, and -9; IL-6, -8, -10, and -12; IFN-γ; TNF-α; and NOS mRNAs in the effusion fluid of both the otitis-prone and non-otitis-prone groups were measured. The expression levels of TLR-2, -4, -6, and -9 mRNA were significantly lower in the otitis-prone than in the non-otitis-prone group (P<0.05). Although higher levels of TLR, cytokine, and NOS mRNAs were generally observed in culture positive than in culture negative patients, none of these differences was statistically significant. No differences were observed in the expressions relative to the frequencies of ventilation tube insertion.
Interpretation & conclusions:
TLRs, cytokines, and NOS, which act cooperatively in the innate immune response, were closely associated with OME. Decreased expression of TLRs may be associated with increased susceptibility to OME.
Keywords
Cytokine
nitric oxide synthase
otitis media with effusion
Toll-like receptors
Otitis media with effusion (OME) is a disease in which secreted fluid accumulates in the middle ear cavity and is a major cause of hearing loss in children1. Although most patients spontaneously recover, some patients show frequent recurrence of otitis media. About 5 per cent of children are otitis-prone, defined as experiencing more than three recurrences of otitis media within six months or more than four per year2. Inflammatory reactions induced by pathogens are regarded as important in understanding the mechanisms of immune response in the middle ear and in treating these patients34.
The first step in the activation of the human defense mechanism against microbes is the recognition of pathogens by macrophages. Macrophages recognize pathogen-associated molecular patterns (PAMPs), generating intracellular signals and producing cytokines and chemokines, leading to the activation of the acquired immune system5. The recognition and reaction of PAMPs is controlled by pattern-recognition receptors (PRRs), including Toll-like receptors (TLRs), which bind to infecting microbes and directly induce innate host defense responses6. The pathogenesis of OME also involves the inflammatory mediator nitric oxide, which mediates the development of OME by increasing vascular extravasation, neutrophil migration, and mucin hypersecretion78. The cytokines, a group of glycoproteins that participate in modulating inflammatory and immune reactions in many diseases, were found to be involved in OME in humans and experimental animals9.
Although the immunologic aetiology and mechanisms of recurrent otitis media have been thoroughly investigated, but the TLRs, cytokines, and NO were evaluated separately. Little is known about how the innate immune system first reacts with pathogens invading the middle ear cavity, or about the combined expression of TLRs, cytokines, and NO during OME. We therefore, studied the expression levels of TLRs, cytokines, and NO and their relationship in patients with OME.
Material & Methods
Study subjects: Effusion fluid samples were obtained from 96 paediatric patients who visited the Department of Otorhinolaryngology, School of Medicine, Kyung Hee University, Seoul, Korea, and who underwent ventilation tube (v-tube) insertion to treat chronic OME between September 2009 and August 2011. Children were enrolled after approval of the study protocol was obtained from the Medical Ethics Committee of Kyung Hee University Hospital; all parents or guardians provided written informed consent.
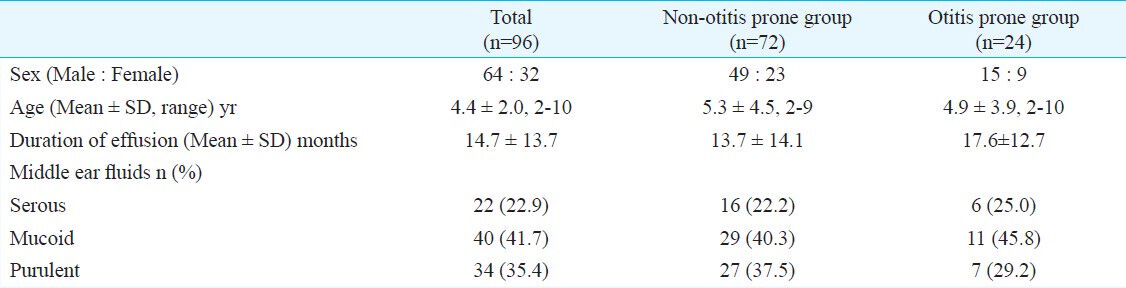
The subject group consisted of 96 paediatric patients (62 males, 32 females) ranging in age from 2-10 yr (mean ± SD age, 4.4 ± 2.2 yr); 72 non-otitis-prone children (49 males, 23 females) ranging in age from 2-9 yr (mean ± SD age, 5.3 ± 4.5 yr); and 24 otitis-prone children (15 males, 9 females) ranging in age from 2-10 yr (mean ± SD age, 4.9 ± 3.9 yr). On evaluating the characteristics of middle ear effusion (MEE), 16 had serous, 29 had mucoid and 27 had purulent MEE in non-otitis-prone children. For otitis-prone group, six had serous, 11 had mucoid and seven had purulent MEE (Table I).
At the first visit, each patient underwent a detailed medical history and physical examinations, including anterior rhinoscopy, otoscopy, impedance audiometry, pure tone audiometry and speech audiometry. OME was diagnosed by the presence of an amber-coloured tympanic membrane on otoscopic examination and by the presence of B- or C-type tympanograms on impedance audiometry. Of these 96 children, 24 had been treated more than four times within the previous year, or more than three times within the previous six months, and were categorized as the otitis-prone group, whereas the other 72 children constituted the non-otitis-prone group. Surgery was performed on patients with chronic OME who did not show improvement after two wk of antibiotic treatment and after a 2-3 month follow up, in patients who showed progressive retraction of the eardrum or progression of hearing loss as shown by continuous increase in pure tone threshold.
Middle ear effusion fluid: When surgery was required, the external acoustic meatus was washed with a potadine solution and a radial incision was made in the anterior inferior quadrant of the tympanic membrane. Effusion fluid was aseptically collected with the aid of Juhn Tym-Tap collectors (Medtronic Xomed; Jacksonville, FL, USA); care was taken to avoid bleeding. Fluid samples were transferred to Eppendorf tubes and stored at -80°C.
Effusion fluid samples, in the original collectors, were sampled using sterile cotton swabs (Xomed Trace Products, Jacksonville, FL, USA); the swabs were submerged in Stuart transport medium. Such samples were used to inoculate solid blood agar and liquid thioglycollate medium (Hangang, Kun-po, Korea). Cultures were incubated for 24 h at 35oC, and bacteria that formed colonies were identified by Gram staining and biochemical testing10.
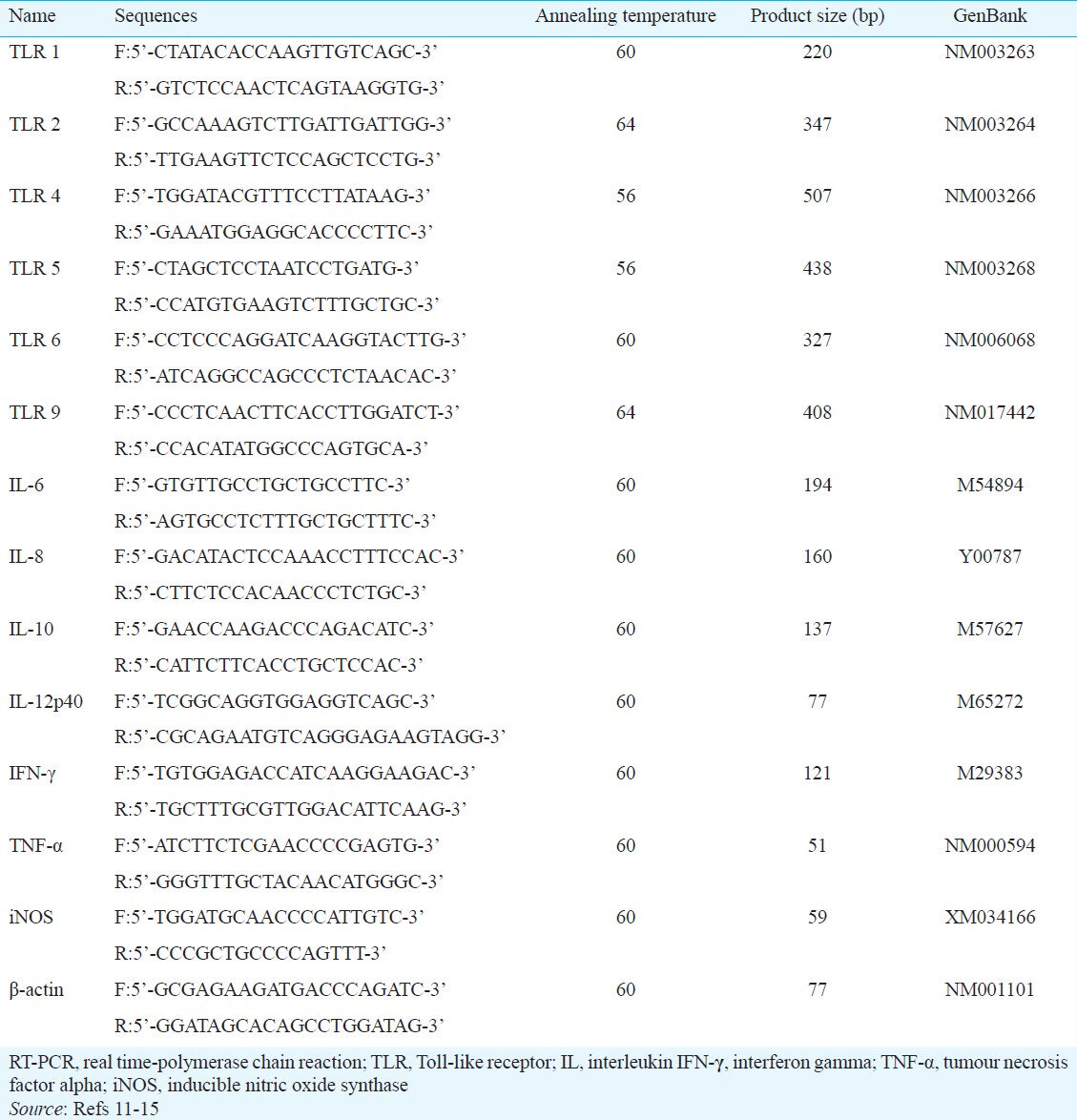
Amplification: Total RNA was extracted from effusion fluid using RNA-Bee solution kits (Tel-Test, Friendswood, TX, USA), according to the manufacturer's protocol. First-strand cDNA was synthesized by reverse transcription in a total volume of 20 μl reaction mixture containing 1 μg of RNA, 1x reaction buffer, 1 mM dNTP, 5 μM random primers, 20 units RNase inhibitor, and 20 units AMV reverse transcriptase (Promega, Madison, WI, USA). The reaction mixture was incubated at 42°C for 1 h, the reaction was terminated by heating at 95°C for 5 min. Primers specific for Toll-like receptors (TLRs) -1, 2, -4, -5, 6, and -9, interleukins (IL)-6, -8, -10, and -12, interferon (IFN)-γ, tumour necrosis factor (TNF)-α, and NOS are shown in Table II1112131415. Real-time polymerase chain reactions (PCR) were performed using a Chromo4 Detector real-time system (Bio-Rad, Hercules, CA, USA) and the SsoFast EvaGreen supermix (Bio-Rad). Each PCR reaction included 2 μl of cDNA in a 20-μl reaction mixture containing 10 μl SsoFast EvaGreen supermix, 2 μl of each primer and 6 μl PCR grade water. The amplification protocols consisted of an initial denaturation at 95°C for 30 sec, followed by 45 cycles of denaturation at 95°C for 5 sec and annealing and extension at 55 to 64°C for 12 sec. The point at which expression of each of the above cDNAs crossed with that for β-actin was applied to the formula, 2-(target gene- ß actin), and the relative amounts were quantitated16.
The level of expression of each mRNA was compared in the otitis-prone and non-otitis-prone groups, in patients with and without bacteria, and by frequency of ventilation tube insertion.
Statistical analysis: The data were analyzed by Mann-Whitney U test using SPSS version 13 (Chicago, IL, USA), with a P value less than 0.05 was considered significant. Pearson's correlation analysis was used to study correlations between the expression levels of TLRs, IL, IFN- γ, TNF- α and NOS mRNA.
Results
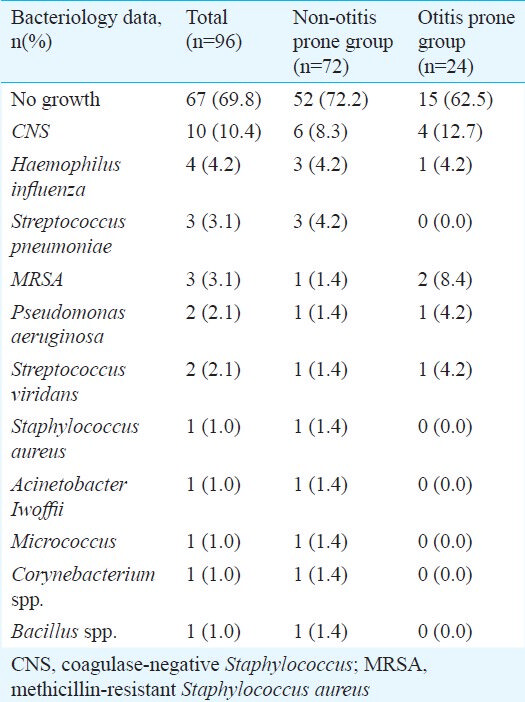
Of the 96 effusion fluid samples examined, 67 (69.8 %) were apparently sterile, whereas bacteria grew from the remaining 29 samples (30.2%). The bacteria detected included coagulase-negative Staphylococcus (CNS), Haemophilus influenzae, Streptococcus pneumoniae, multhicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, Streptococcus viridans, Staphylococcus aureus, Acinetobacter lwoffii, Micrococcus sp., Corynebacterium sp., and Bacillus sp. (Table III).
The expression of TLR-1, -2, -4, -5, -6, and -9; IL-6, -8, -10, and -12; IFN-γ; TNF-α; and NOS mRNAs in the effusion fluid of both the otitis-prone and non-otitis-prone groups was measured. The expression levels of TLR-2, -4, -6, and -9 mRNA were significantly lower in the otitis-prone than in the non-otitis-prone group (P<0.05) (Fig. 1 and 2).
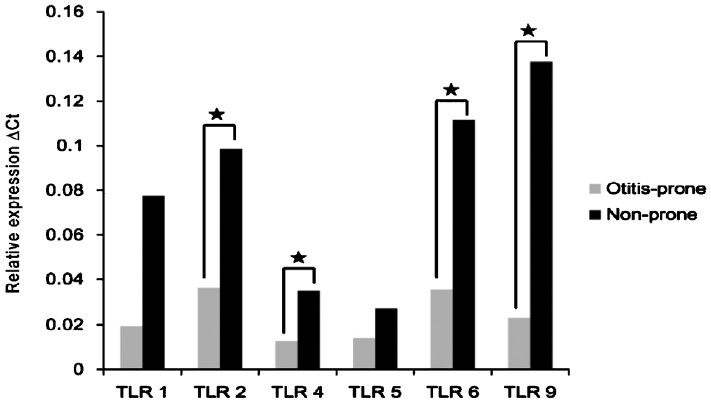
- TLR mRNA expression in the effusion fluid of the otitis-prone and non-otitis-prone groups. ΔCt, threshold cycle; *=P<0.5.
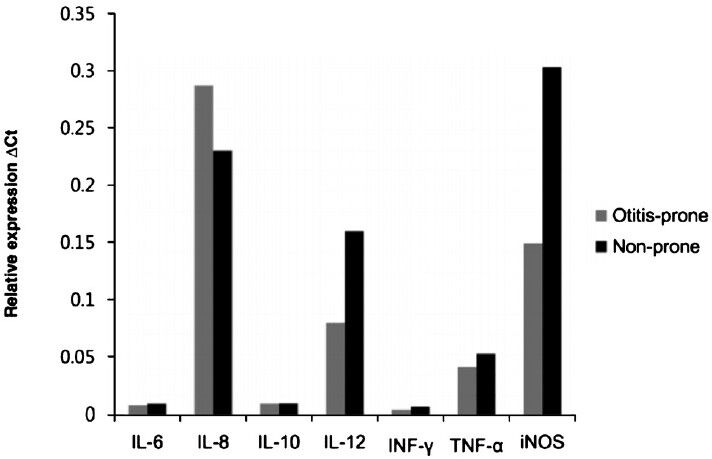
- Cytokine and NOS mRNA expression in the effusion fluid of the otitis-prone and non-otitis-prone groups.
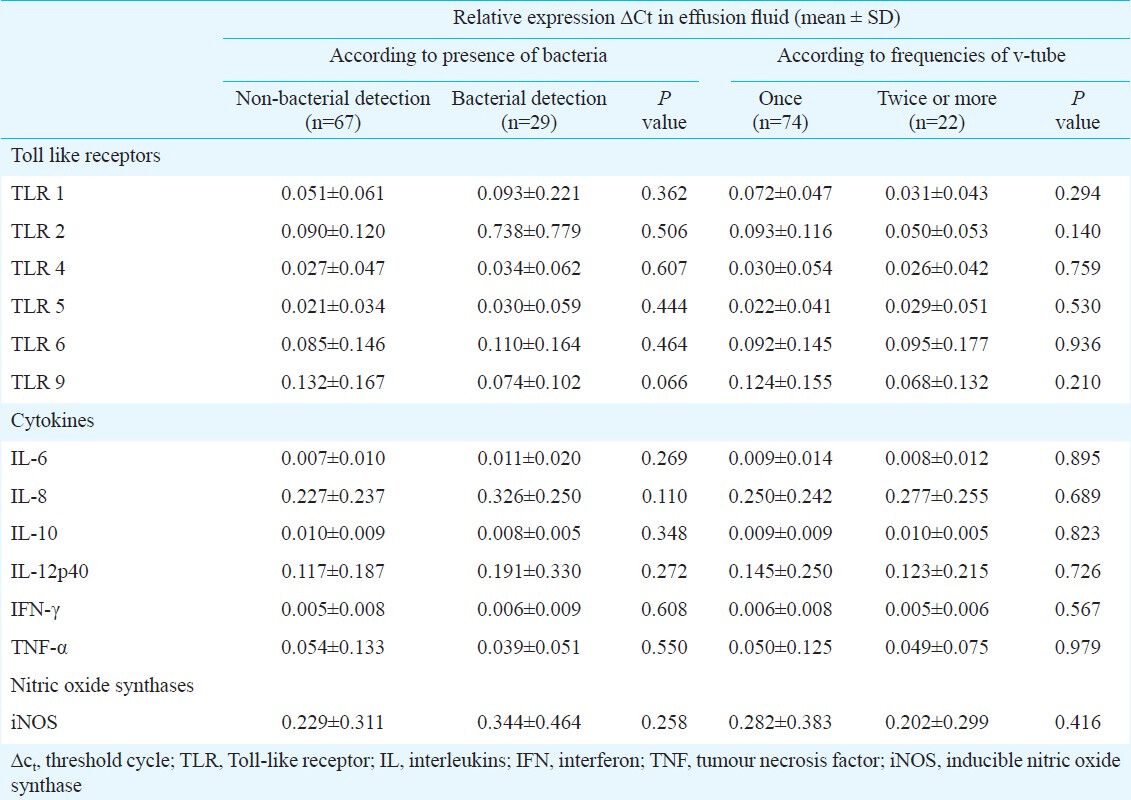
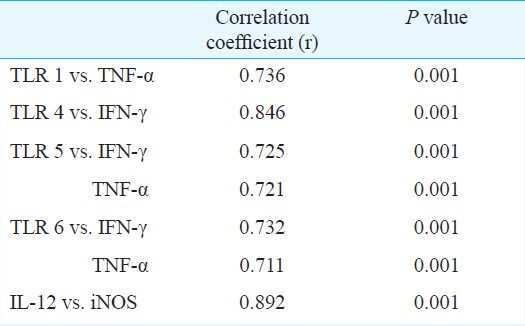
Although higher levels of TLR, cytokine, and NOS mRNAs were generally observed in culture positive than in culture negative patients, none of these differences was statistically significant. No differences were observed in the expressions relative to the frequencies of v-tube insertion (Table IV). The expression levels of these mRNAs were correlated (Table V).
Discussion
Otitis-prone children showed significantly lower expression levels of mRNAs encoding TLR-2, -4, -6, and -9 than did those who were not otitis-prone, indicating that reduced TLR expression in the middle-ear cavity may increase susceptibility to recurrent OME.
Of the various relevant factors, TLR2 and TLR4, in particular, are known to play important roles in molecular pathogenesis and in the development of host defenses to otitis media171819. An experimental study suggested that decreased production of proinflammatory cytokines by virtue of defective TLR2 functionality in those with acute otitis media might hinder bacterial clearance from the middle ear20. We previously reported that the expression level of TLR9 was significantly lower in an otitis-media-prone group; a similar trend was evident in the present study6. It has also been reported that the otitis-prone condition is associated with certain genetic polymorphisms, especially in genes involved in the innate immune response. Such alleles include TNFA-863A, TNFA-376G, TNFA-238G, IL10-1082A, and IL6-174G; the variant alleles differ in the promoter regions21.
Bacteria are potent triggers of monocyte/macrophage cytokine secretion as well as TLR expression. Gram-positive bacteria induce the secretion of IL-12, TNF, and IFN-γ, whereas Gram-negative bacteria induce the secretion of IL-6 and IL-1022. TNF-α is central for extravasation of polymorphonuclear leukocytes into infected tissue. IL-12, IFN-γ, and TNF-α are key cytokines in cell mediated immune reactions23. In addition, TNF-α plays a role in prostaglandin and cytokine release, as well as in the activation of neutrophils, eosinophils, and macrophages. TNF-α has many of the same functions as IL-1, with the two having a synergistic effect24. IL-6 activates B and T cells, resulting in the production of antibodies, and the induction of fever and bone resorption. IL-6 expression is significantly correlated with OME and degree of hearing loss25. IL-10 downregulates the inflammatory properties of IL-1, IL-6, and TNF-α and is thought to contribute to the eradication of middle ear inflammation as well as providing a negative feedback mechanism related to TNF-α in patients with otitis media2627. IL-12 is the major cytokine responsible for the differentiation of TH1 cells, which produce IFN-γ. IFN-γ activates macrophages and NK cells, and potentiates the proliferation of activated T cells28.
In our study, the levels of expression of all the cytokines tested, IL-6, -8, -10, and -12; IFN-γ; and TNF-α did not differ significantly between otitis-prone and non-otitis-prone groups. Effusion in the middle ear is a chronic state, with inflammation occurring for at least three months. Thus, effusion fluid, in contrast to middle ear mucosa, could not fully reflect inflammatory reactions in this organ.
Nitric oxide is responsible for vasodilation, increased vascular permeability, and production of mucoid effusion in patients with otitis media29. Incubation of middle ear epithelial cells with IL-1 ß or TNF-α induced NO, both in vivo and in vitro, suggesting that NO may be a secondary mediator of inflammation produced by middle ear epithelium in response to primary proinflammatory cytokines30.
This study had several limitations. We did not include any children with early stage OME. In addition, all patients had been treated with antibiotics for two week for early stage symptoms. Third, 69.8 per cent of the middle ear samples were negative for bacterial growth despite bacterial infection, which could have been due to treatment with antibiotics before surgery, creating a bacteriostatic condition and delaying the proliferation and growth of pathogens. Fourth, the exudates used in this study were collected during surgery 2-3 months after the initial onset of otitis media. Thus, our findings may not fully reflect the initial immune response in the middle ear cavity. Moreover, though our study group included patients with OME, all patients had normal immunity, and both otitis-prone and non-otitis prone patients had chronic effusions in the middle ear cavity without improvement. Furthermore, for ethical reasons, we could not harvest the middle ear mucosa from the patients in this study and performed experiments on exudates. These exudates contained only a few partially exfoliated mucosal epithelial cells and inflammatory cells; therefore, these would not fully reflect the immune cells present in the middle ear mucosa during infection. Finally, we could not determine the expression levels of TLR, cytokine, and NOS mRNAs at the initial time of otitis media, but only in fluid secreted after inflammatory reactions. Therefore, our results may reflect more complex anti-infective mechanisms occurring in the middle ear cavity. Therefore, our results eflect the situation obtained when complex anti-infective mechanisms are triggered in the middle-ear cavity, and thus need to be interpreted with caution.
In conclusion, our findings showed that TLRs, cytokines, and NOS worked cooperatively in innate immune responses and were closely associated with OME. However, all exudates of OME patients showed some level of TLR expression related to the immune response, regardless of the presence of the bacteria in exudates, or the frequency of ventilation tube insertion. Thus different levels of expression of TLRs may be important indicators of immune responses in patients with OME, and decreased expression of TLRs may be associated with increased susceptibility to OME.
Acknowledgment
This research was supported by the Kyung Hee University Research Fund, Korea, in 2011(KHU-2011-0899).
References
- Otitis media with effusion: Frequency, diagnosis and therapy in early childhood. HNO. 2013;61:859-65.
- [Google Scholar]
- Postnatal development of the Eustachian tube and its surrounding structure. Ann Otol Rhinol Laryngol. 1987;96:191-8.
- [Google Scholar]
- Relationship between effusion bacteria and concentrations of immunoglobulin in serum and effusion fluid in otitis media with effusion patients. Int J Pediatr Otorhinolaryngol. 2008;72:337-42.
- [Google Scholar]
- Host's response in otitis media: understanding genetic susceptibility. Pediatr Infect Dis J. 2008;27:929-33.
- [Google Scholar]
- Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975-9.
- [Google Scholar]
- TLR-9, NOD-1, NOD-2, RIG-I and immunoglobulins in recurrent otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2010;74:1425-9.
- [Google Scholar]
- Signaling events induced by lipopolysaccharide-activated toll-like receptor 2. J Immunol. 1999;163:639-43.
- [Google Scholar]
- Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736-9.
- [Google Scholar]
- Cytokines in children with otitis media with effusion. Eur Arch Otorhinolaryngol. 2000;257:323-6.
- [Google Scholar]
- IgA and differentiation-associated transcription factors in chronic otitis media with effusion. Clin Exp Otorhinolaryngol. 2009;2:131-5.
- [Google Scholar]
- Corticosteroid and cytokines synergistically enhance Toll-like receptor 2 expression in respiratory epithelial cells. Am J Respir Cell Mol Biol. 2004;31:463-9.
- [Google Scholar]
- Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: implications for survival. Cancer Immunol Immunother. 2005;55:684-98.
- [Google Scholar]
- mRNA expression and localization of bNOS, eNOS and iNOS in human cervix at preterm and term labour. Reprod Biol Endocrinol. 2005;3:33.
- [Google Scholar]
- Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol Immunol. 2007;44:3100-11.
- [Google Scholar]
- Guggulsterone suppresses LPS induced inflammation of human middle ear epithelial cells (HMEEC) Int J Pediatr Otorhinolaryngol. 2010;74:1384-7.
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta delta C(T)) method. Methods. 2001;25:402-8.
- [Google Scholar]
- Toll-like receptors 2 and 4 and their mutations in patients with otitis media and middle ear effusion. Clin Exp Otorhinolaryngol. 2008;1:189-95.
- [Google Scholar]
- Differential expression of toll-like receptors 2 and 4 in rat middle ear. Int J Pediatr Otorhinolaryngol. 2009;73:821-4.
- [Google Scholar]
- Innate signaling in otitis media: pathogenesis and recovery. Curr Allergy Asthma Rep. 2011;11:78-84.
- [Google Scholar]
- Toll-like receptor 2 and Toll-like receptor 4 participates in mediation of acute otitis media and mortality in pneumococcal infections in mice. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46:1009-18.
- [Google Scholar]
- Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics. 2007;120:814-23.
- [Google Scholar]
- Gram-positive and Gram-negative bacteria induce different patterns of cytokine production in human mononuclear cells irrespective of taxonomic relatedness. J Interferon Cytokine Res. 2010;30:23-32.
- [Google Scholar]
- Interleukin-1beta upregulates Na+-K+-2Cl- cotransporter in human middle ear epithelia. J Cell Biochem. 2007;101:576-86.
- [Google Scholar]
- Interleukin-1 induces tumor necrosis factor (TNF) in human peripheral blood mononuclear cells in vitro and a circulating TNF-like activity in rabbits. J Infect Dis. 1990;162:215-23.
- [Google Scholar]
- Middle ear epithelial mucin production in response to interleukin-6 exposure in vitro. Cytokine. 2004;26:30-6.
- [Google Scholar]
- Evidence of T-helper cell 2 cytokine regulation of chronic otitis media with effusion. Acta Otolaryngol. 2005;125:1043-50.
- [Google Scholar]
- Cytokine profiles in a rat model of otitis media with effusion caused by eustachian tube obstruction with and without Streptococcus pneumoniae infection. Laryngoscope. 2002;112:1657-62.
- [Google Scholar]
- The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolaryngol. 2008;1:117-38.
- [Google Scholar]
- Glucocorticoids reduce nitric oxide concentration in middle ear effusion from lipopolysaccharide induced otitis media. Int J Pediatr Otorhinolaryngol. 2010;74:384-6.
- [Google Scholar]
- Expression of inducible nitric oxide synthase (iNOS) in middle ear epithelial cells by IL-1beta and TNF-alpha. Int J Pediatr Otorhinolaryngol. 2000;55:91-8.
- [Google Scholar]






