Translate this page into:
CD4 estimating reagents in dry format are compatible with conventional flow cytometer; FACSCalibur for estimation of absolute CD4 count & percentages
Reprint requests: Dr Madhuri Thakar, National AIDS Research Institute (ICMR), Pune 411 026, India e-mail: mthakarr@hariindia.org
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Reliable CD4 counts are important for successful implementation of antiretroviral treatment (ART). Availability of dry CD4 reagents can eliminate cold chain requirement reducing shipment and storage cost. An attempt was made in this study to validate the ReaPan and Rea T Count dry reagents developed by ReaMetrix against the original BD Biosciences liquid reagents.
Method:
Absolute counts and percentages of CD4, CD8 and CD3 + T cells obtained in 100 HIV infected individuals using the test and reference reagents were analyzed for correlation and agreement using Pearson's correlation and Bland Altman bias analysis. The stability of the reagents and of the stained samples was analyzed at ambient temperature and at 37°C.
Results:
The absolute CD4 + T cell count and percentages obtained using test and reference reagents showed correlation coefficients ranging from 833 to 981. A mean bias between dry and reference reagents ranged from 0.8 to 26.4. The ReaPan and Rea T Count reagents were stable up to one month at 37°C also. The samples stained with ReaPan reagents were stable at ambient temperature till day 7 whereas the samples stained with Rea T Count reagents were stable at ambient temperature and at 37°C for 10 days.
Interpretation & conclusions:
The ReaPan dry reagents can be used on existing FACSCalibur machines with additional training on Cell Quest Pro software without incurring any additional equipment cost and this can eliminate the requirement of cold chain during transport and on site storage. The stability of the stained samples has great clinical significance preventing redrawing of the blood samples from the patients.
Keywords
CD4
dry reagents
FACSCount
flow cytometry
HIV
quality control
stability
HIV infection continues to be a major health problem worldwide with 33.3 million people living with HIV at the end of 20111. The scale up of antiretroviral treatment (ART) spearheaded by World Health Organization (WHO) has ensured availability of ART to 8 million people in low- and middle-income countries2. India had an estimated 2.4 million persons living with HIV in 2009, with an estimated adult HIV prevalence of 0.31 per cent3. Approximately 486,000 of them were on ART by January 20114.
Public Health programmes in resource-limited settings rely on CD4+ cell count estimations for decisions related to initiation of ART as well as treatment monitoring. Hence availability of facilities providing reliable estimates of CD4+ T cell counts is of utmost importance for successful implementation of ART programmes. Flow cytometry (FCM) is the reference standard for estimation of CD4+ T-lymphocyte counts, because of its accuracy, precision, and reproducibility56. The FACSCalibur; a conventional flow cytometer and FACSCount, a dedicated flow cytometer for CD4+, CD8+, CD3+, T cell count estimation (both from Becton Dickinson, USA) are the most widely used equipment worldover5–8. However, the fluorescent labelled antibodies still need to be shipped and stored at 2-4°C. This poses additional challenges especially in the resource limited settings, of maintaining cold chain during transport and storage. It also increases chances of inaccurate CD4+ count estimations or non availability of CD4+ cell counts due to malfunctioning of the reagents.
Dry reagents that do not require cold chain and are compatible with FACSCount and FACSCalibur have been developed and marketed by ReaMetrix, Bengaluru, India, as Rea T Count and ReaPan, respectively9. These reagents could potentially reduce costs of shipment and storage. Use of such reagents would help in making the CD4 counts available at a reduced cost in areas where the FACSCount and FACSCalibur machines are already in place and where maintenance of cold chain is a challenge. While the Rea T Count reagents have already been evaluated against the FACSCount liquid reagents10; their stability at ambient temperature and at 37 ° C has not been assessed. The ReaPan reagents have not been assessed for their compatibility with FACSCalibur. This study evaluated ReaPan and Rea T Count dry reagents against the respective reference standards; MultiTEST liquid reagents with TruCOUNT tubes and FACSCount liquid reagents, respectively. The stability of these reagents at ambient temperature (20 to 25°C) and at 37°C was also assessed. In addition, the samples stained with these dry reagents were also assessed for their integrity and stability at ambient temperature as well as at 37°C. Further, the ability of these reagents to correctly identify samples with CD4 counts less than 350 cells/μl was assessed as in India the ART is initiated in the HIV infected patients with the CD4 count less than 350 cells/μl and it was important to estimate the misclassification of the samples to correctly assist in ART initiation.
Material & Methods
Study population: The study was conducted from May to August 2009. During this period, every day 5 to 7 HIV positive patients attending the outpatient clinics at National AIDS Research Institute, Pune, India, were enrolled consecutively after obtaining informed consent. The inclusion criteria included age between 18 to 60 yr and willingness to give blood sample for CD4 count estimation. The exclusion criteria included serious medical condition such as disseminated tuberculosis, other autoimmune condition and malignancies which might interfere with the accuracy of the testing. One hundred HIV infected patients (M=46 and F54), with the age ranging between 18 to 54 yr were enrolled in the study after obtaining written informed consent for CD4 count estimation. The CD4 count of these patients ranged from 47 to 1027 cells/μl. The study protocol was approved by the institutional ethical committee of the National AIDS Research Institute.
Sample processing: Whole blood samples (3 ml) were collected in EDTA vacutainers and processed within 24 h of collection. In the laboratory, the samples were divided into four aliquots. These aliquots were coded to avoid cross matching of the results during processing. The samples were decoded only when testing of all 100 samples was completed. The samples were tested by personnel not involved in the coding.
Sample processing for FACSCalibur MultiTEST liquid reagents with TruCOUNT tubes: Twenty μl of liquid antibody reagent (MultiTEST CD3 FITC, CD8 PE, CD45 PerCP and CD4 APC, Cat no.340491, Becton Dickinson, USA) and 50 μl of whole blood was added to the TruCOUNT tube (Cat No: 340334, Becton Dickinson) containing the reference beads. The tube was vortexed and incubated at ambient temperature in dark for 15 min. The RBCs were lysed using 450 μl of 1:10 diluted lysing solution (FACSlysing, BD) for 15 min in the dark at ambient temperature. The stained sample was acquired on the FACSCalibur and analyzed using the automated MultiSET software by automated gating. After acquiring data up to 1,000,00 events per sample, a region (R1) was automatically set on the lymphocyte cluster (SSC-Hlow/CD45 PerCP high+ cells) (Fig. 1A, plot 1). The CD3+ T cells from the CD45+ cells were differentiated into double positive (CD3+/CD8+) T cells in upper right region and the CD3+CD8- cells i.e. CD4+ T cells in lower right region(Plot 2). The third plot is used to gate the CD3+ T cells which are further differentiated into CD4+ (upper left quadrant) and CD8+ T cells (lower quadrant)(plot 4). Per cent and absolute counts of CD4+, CD3+, CD8+ T lymphocytes were then generated by the MultiSET software.
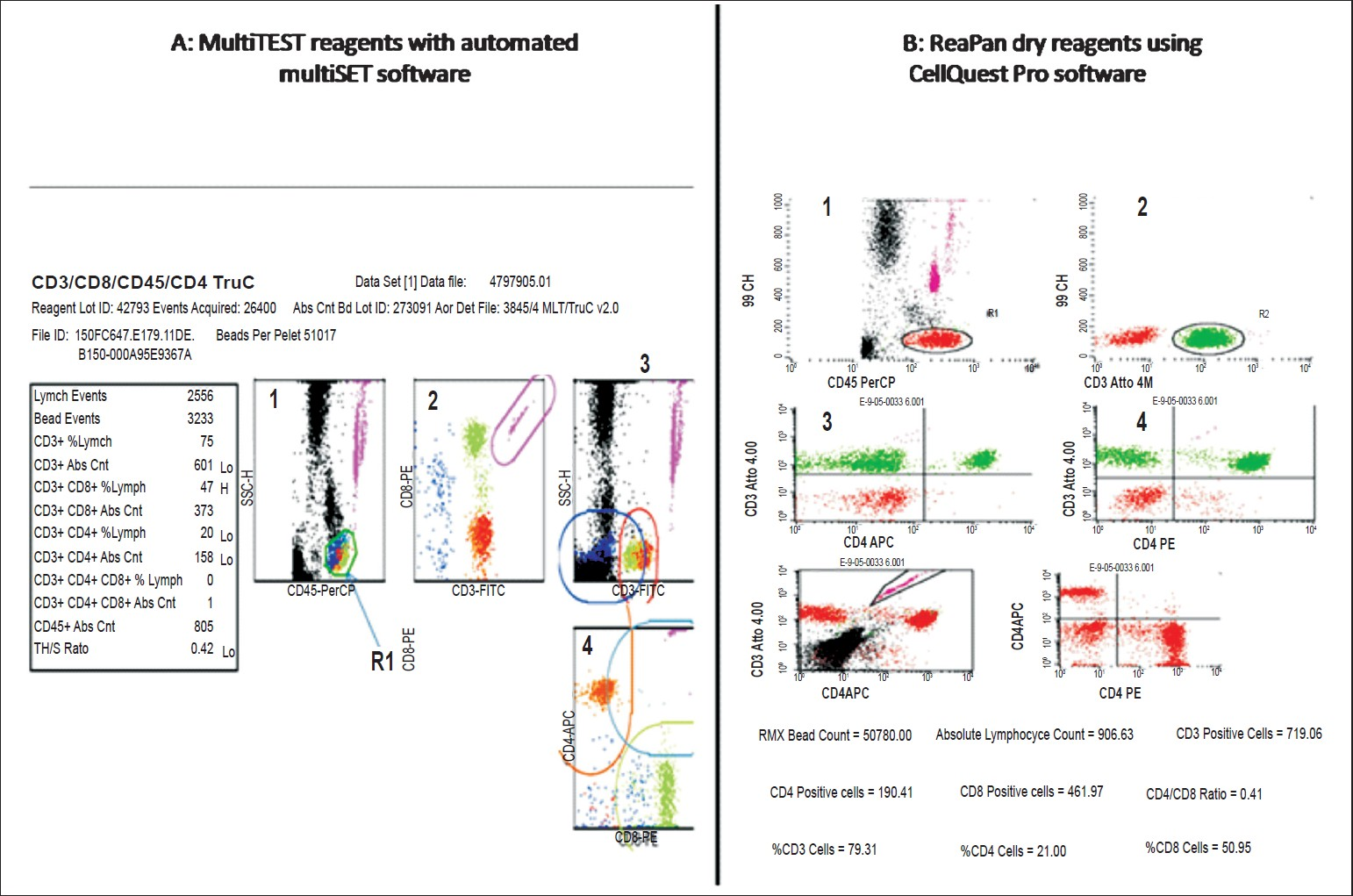
- Representative flwo cytometric dot plot display for the reference (Fig. 1A) and the test reagents (Fig. 1B). The plot have been numbered as 1, 2, 3 serially.
ReaPan dry reagents: Fifty μl of whole blood was added to the ReaPan dry reagent tube containing preadded reference beads and antibodies (CD45 PerCP, CD3 Atto488, CD4 APC and CD8 PE) (ReaPan 34845 Cat no: 25239-00:, ReaMetrix, India) in a dry form. The tube was vortexed to ensure proper mixing of the sample and the dry reagents and incubated at ambient temperature in the dark for 30 min. The RBCs were lysed using 450 μl of 1:10 diluted lysing solution (ReaLyse, ReaMetrix, India) for 15 minutes in the dark at ambient temperature. The stained sample was acquired on the FACSCalibur and analyzed using the Cell Quest Pro software
The algorithm of the dot plots used for analysis is given in Fig. 1B. The number of beads acquired in the preset gate and 3000 beads events were acquired for each sample (Fig. 1B, plot 5,gate R3). The CD45 vs SSc gate is used to acquire all the leucocytes (plot 1, gate R1,). The second plot captures the CD3+ T lymphocytes (gate R2) from the CD45+ cells The gated cD3+ t cells were then further classified as CD3+CD4+ and CD3+ CD8+ cells in the upper right corners of the dot plots of CD3Atto488 and CD4 APC(plot 3) or CD3Atto 488 and CD8PE(plot 4). The sixth dot plot of CD4APC and CD8PE was used to confirm the percentages of CD4+ and CD8+ T cells obtained from third and forth plots. The per cent CD4+, CD3+, CD8+ T lymphocytes was calculated by the software Absolute CD4/CD3/CD8 counts were calculated by the following formula:
Absolute count/μl= (Gated count) × bead count/test)/ (gated bead count) × (test volume)
Where the gated cell count and bead count were obtained from the flow cytometer analysis and the bead count per test from the test kit provided.
Sample processing using FACSCount: FACSCount liquid reagents: Fifty μl of whole blood was added to both FACSCount reagent tubes containing either CD3 PE /CD4 PE. Cychrome (PE.Cy5) or CD3 PE/CD8 PE.Cy5 monoclonal antibodies and a known number of reference beads in a liquid format (Cat No 340167, Becton Dickinson, USA). The tubes were vortexed and incubated for 60 to 120 min in the dark at ambient temperature. Fifty μl of fixative solution (5% formaldehyde in PBS) was added. The stained sample was acquired and analyzed on the FACSCount (software version 1.2) within 24 h of staining. After acquisition of 30,000 events for each sample, the gate was set up automatically around CD3+/CD4+ or CD3+/CD8+ T lymphocytes.
The absolute number of CD3+/CD4+ and CD3+/CD8+ T lymphocytes was calculated automatically.
Rea T Count dry reagents: To the Rea T Count reagent tubes (containing either CD4 PE /CD3PE.Dyomics649 or CD8 PE/CD3 PE.Dyomics649 monoclonal antibodies and a known number of reference beads in dry form) (Cat No 25124-00, ReaMetrix, India), 50 μl of whole blood was added. The tubes were vortexed vigorously to ensure proper mixing of the dry reagents with the blood samples and incubated for 30 min at ambient temperature in the dark. Four hundred and fifty μl of fixative (ReaFix) was added to the tubes. The stained sample was acquired and analyzed on the FACSCount (software version 1.2) within 24 h of staining using the procedure mentioned above.
Quality control: As part of quality control procedures for FACSCalibur, the photomultiplier tube voltage, sensitivity and fluorescence compensation settings were optimized using calibrate beads (Becton Dickinson, USA) with FACSComp software on each day before running the samples using channel targeting technique11. For the FACSCount, the BD control beads at zero, low, medium and high, were acquired along with whole blood with normal CD4 count to ensure that the equipment was calibrated successfully before running the samples each day.
Additionally, commercially available stabilized blood samples; Immuno-Trol (Low & Normal level, Beckman Coulter, USA) were included as internal quality control in the staining and acquisition along with the samples in each batch. The run was considered valid only when the variation between two consecutive values of Immuno-Trol was less than 10 per cent.
Assessment of precision & Stability assessment: The precision of the ReaMetrix dry reagents was assessed using 10 replicates of each low and normal level Immuno Trol controls stained with ReaPan and Rea T Count dry reagents, respectively. Stability of the dry reagents at ambient temperature (20-25°C); a recommended temperature by the manufacturer and at 37°C was assessed. The low and normal level Immuno-Trol controls were stained with ReaPan and Rea T Count dry reagents stored for 0, 4, 7, 15 and 30 days at both temperatures.
For assessments of stability of stained samples, 10 aliquots of 10 randomly selected samples were stained with ReaPan and Rea T count reagents. One aliquot was analyzed the same day while the remaining aliquots were stored at ambient temperature and at 37°C before analysis. One aliquot stained with each of the two reagents and incubated at ambient and at 37°C was acquired on FACSCalibur and FACSCount respectively on days 2, 4, 7 and 10.
Statistical analysis: The MultiTEST liquid reagents with TruCOUNT tubes and FACSCount liquid reagents were used as the reference reagents for ReaPan and Rea T Count dry reagents, respectively. Pearson's correlation coefficient was used to calculate the strength of the correlation between the values obtained using reference and test reagents. P<0.01 was considered to be significant. The degree of agreement between the values obtained by all reagents was estimated using Bland-Altman analysis and the mean relative bias, line of agreement (LOA) were estimated12. Additionally, the relative biases of the averages between the methods were also calculated13. The percentage similarity values from data pairs of the test reagents compared with the reference standard) were calculated with the formula; [({a+b}/2)/a] × 100, where‘a’ is the reference standard and ‘b’ is the method under evaluation14. The precision was assessed for variation using the values obtained for the Immuno-Trol controls. Per cent CV (%CV) was calculated to assess the stability of the dry reagents and the stained blood samples at ambient temperature and at 37°C. The CV less than 10 per cent was considered to be an acceptable variation for the single platform technology15.
Results
The operational precision of both ReaPan and Rea T Count dry reagents was determined using 10 replicates of each low and normal level Immuno Trol controls. For ReaPan reagents the mean %CV was 4 per cent (range: 2 to 5.1%) for the low cell control and 2.5 per cent (1 to 3.6%) for the normal cell control for all parameters. For Rea T Count reagents the % CV ranged between 2 to 5.4 per cent for all parameters. The precision of reference reagents was comparable with the test reagents with slightly higher %CV. The mean %CV was 5.4 per cent (range: 2.8 to 8.9) for FACSCount reagents and 4 per cent (range: 1.4 to 8.7) for MultiTEST reagents for all parameters for both normal and the low Immuno Trol control. The between run reproducibility assessment was carried out using the CD4 count values of the low and the normal cell controls stained with ReaPan and Rea T Count in 10 consecutive runs. The %CV was found to be below 5 per cent.
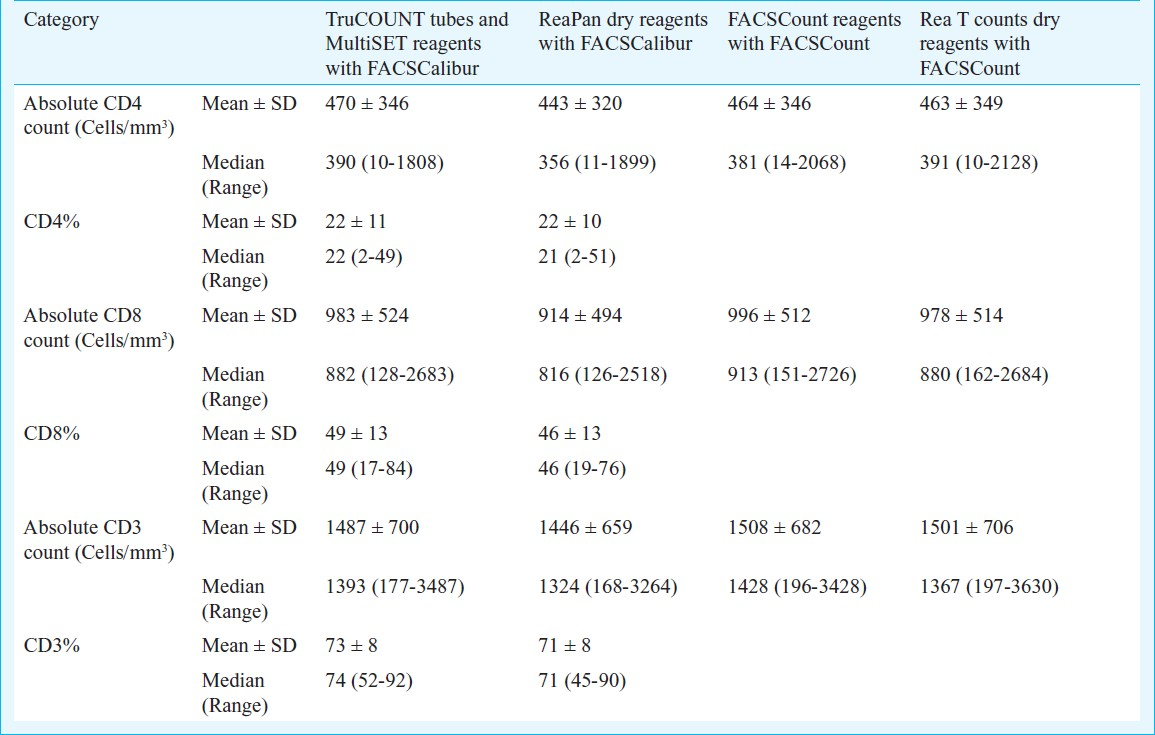
Agreement between Lymphocyte subset data obtained using the reference and test reagents: The mean absolute counts and percentages for CD4+ T cells, CD3 + T cells and CD8 + T cells obtained in 100 patients using all four reagents have been given in Table I. The mean absolute CD4 counts were 470 (median 390) and 443 (median 356) cells/μl using MultiTEST reagents with TruCOUNT tubes and ReaPan dry reagents, respectively. When the FACSCount liquid reagents and Rea T Count dry reagents were compared, the mean absolute CD4 counts were 464 (median 381) and 463 (median 391) cells/μl, respectively (Table I). The mean absolute CD8 and CD3 counts ranged from 914 to 996 (median range 816-913) cells/ μl and 1446-1508 (median range: 1324-1428) cells/ μl respectively (Table I).
Agreement between methodologies using MultiTEST liquid reagents with TruCOUNT tubes and ReaPan dry reagents: Different software were used for analysis of the acquired samples stained with ReaPan and MultiTEST reagents. The MultiSET software is automated software and thus has a preset gate for bead attractor. The location of the fluorescent absolute counting beads was found to be different when the ReaPan reagents were used for testing. Since the sample volume acquired for analysis is dependent on the number of bead events acquired; the cell count would have differed if the MultiSET software has been used. Such differences were seen (data not shown). Hence, the Cell Quest software was used for the acquisition and analysis of the samples stained with ReaPan reagents. The display of both MultiSET and Cell Quest Pro analysis of a representative sample stained with MultiTEST and ReaPan reagents respectively is shown in Fig. 1. The fluorescence intensity of CD4+ and CD8+ population was comparable, although the reference bead position was clearer in MultiSET display (Fig. 1A, plot 1). In the Cell Quest Pro display, the reference beads appeared to be resolved in two different populations in SSC and CD45 PerCP display (Fig. 1B, plot 1), however, in the FL1 versus FL2 display which is used for getting the reference beads, the beads appeared to be very well separated (plot 5).
High correlation was observed for the absolute CD4 counts (r2= 0.969, P<0.001, Table II) and CD4% (r2 = 0.981, P<0.001, Table II) obtained using MultiTEST reagents with TruCOUNT tubes and ReaPan dry reagents. The mean % similarity and % CV were 99 and 9 per cent respectively for absolute CD4 count where as for CD4% the mean % similarity was 98 per cent and the mean %CV was 5 per cent (Table II). The absolute CD8 and CD3 counts obtained using reference liquid and dry reagents also showed high correlation (P<0.001 for all parameters, Table II).
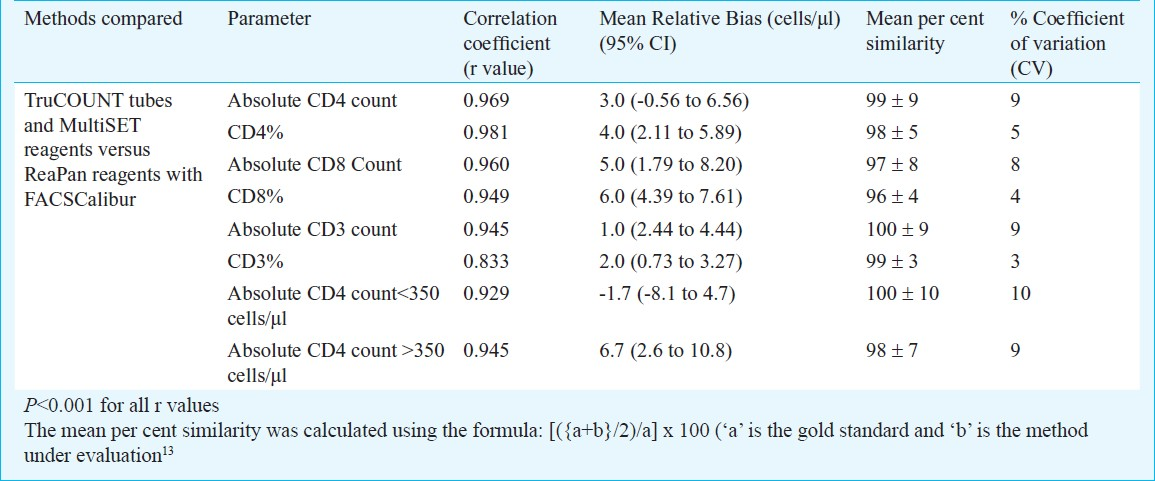
The Bland-Altman plot analysis was carried out to understand the limits of agreement. The mean absolute bias for absolute CD4 count was found to be 26.4 cells/μl (CI: 8.9 to 43.8) (LOA: -146.6 to 199.3) (Fig. 2A), indicating that the ReaPan reagents yielded slightly higher CD4 counts than the MultiTEST reagents. The mean bias was only 0.8 (CI: 0.4 to 1.2) (LOA-3.3 to 4.9) (Fig. 2B) for CD4% showing excellent agreement. The Bland Altman analysis for absolute counts showed higher mean relative bias of 61.8 for absolute CD8 (Fig. 2C) and 41.3 for CD3 (Fig. 2E) counts. The mean bias for percentages of CD8 and CD3 T cells was found to be low, 3.3 (CI: 2.5 to 4.1) and 1.6 (CI: 0.6 to 2.6) respectively (Fig. 2D and 2F). The mean relative biases ranged between 1 to 6 per cent which were comparable to within run coefficient of variation of 5 per cent showing excellent agreement between reference and test reagents (Table II).
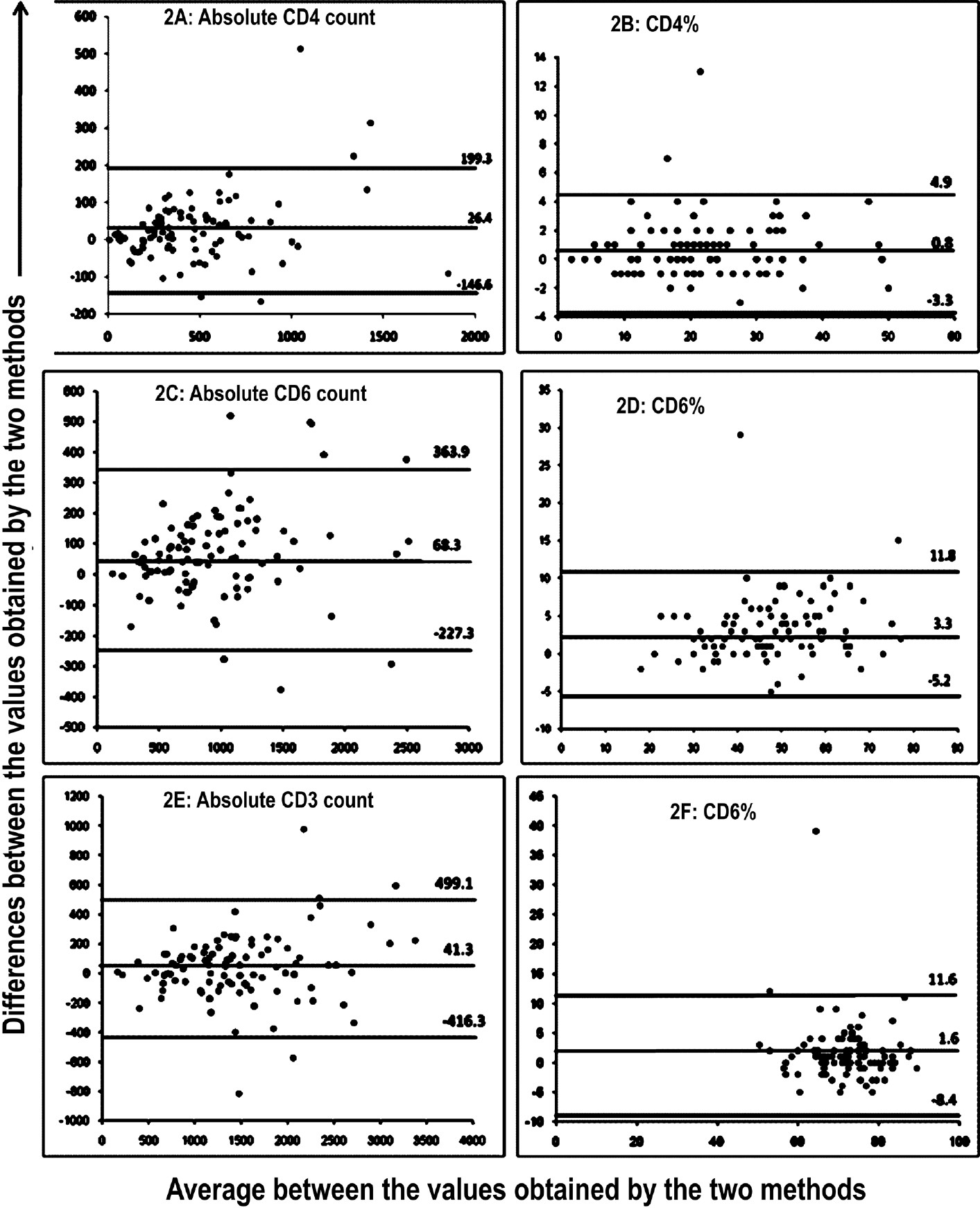
- The Bland Altman (ba) bias plots for the absolute counts and percentages of CD4, CD8 and CD3+ T cells obtained using TruCOUNT and reapan reagents. Figure 2A, 2C, 2E show the BA plots for absolute CD4, CD8 and CD3 counts and figures 2B, 2D and 2F show the BA plots for CD4, CD8 and CD3 percentages, respectively. The X axis shows the average between the values obtained by the two methods and the Y axis shows the differences between the values obtained by the two methods.
Additionally, the agreement between MultiTEST liquid reagents with TruCOUNT tubes and ReaPan dry reagents was assessed separately for absolute CD4 counts <350 cells/μl and CD4 counts >350 cells/μl.
For all CD4 counts, the r2 and % similarity values were >0.9 and >95 per cent, respectively (Table II). For absolute CD4 counts <350 cells/μl and >350 cells/μl the mean relative bias were found to be very low -1.7 cells/μl, (95% CI: -8 to 4.7) for CD4 counts <350 and 6.7 cells/μl (95% CI: 2.6 to 10.8) for CD4 >350.
Agreement between Rea T Count dry reagents and the FACSCount reagents: The dot plots of the samples stained with FACSCount and Rea T Count reagents showed that fluorescence intensity of CD3+CD4+ and CD3+CD8+ clusters was comparable and the position of reference bead cluster was identical (data not shown). High correlation was observed between the FACSCount and Rea T Count reagents (r2= 0.982, 0.974 and 0.974 for absolute CD4, CD8 and CD3 counts respectively, P<0.01). The mean % similarity for absolute CD4, CD8 and CD3 counts was 100 ± 5, 106 ± 8, 102 ± 9 with %CV of 5, 7 and 9 per cent, respectively (Table II).
Mean relative bias of -0.1 (95% CI: -1.95 to 2.15) and 1.0 (95% CI: -0.473 to 2.47) were observed for absolute CD4 and CD3 counts whereas, a negative bias of -18.5 was observed for absolute CD8 counts. For the samples with absolute CD4 counts <350 cells/μl, the Rea T Count dry reagents showed a small negative bias of -0.4 (95% CI: -3.5 to 2.71) with a coefficient of correlation of 0.987 and % similarity of 100 ± 5. For samples with absolute CD4 count>350 cells/μl, the mean relative bias was 0.5 (95%CI: -2.77 to 3.68) with an r2 value of 0.984 and 100 ± 5 per cent similarity.
Stability of the ReaPan and Rea T Count dry reagents: Stability of the dry reagents and also of the samples stained with the dry reagents was assessed at ambient temperature (20-25 ° C) and at 37°C.The %CV for low and normal Immuno Trol controls stained with the ReaPan dry reagents stored at the recommended storage temperature, i.e. ambient temperature was below 5 per cent. The %CV calculated over time (till day 30) for storage at ambient temperature and 37°C were also below 5 per cent for all parameters. Whereas the for Rea T Count reagents the %CV was little higher ranging from 4 to 8 per cent for all parameters. Table III shows the %CV for the absolute CD4 counts from the low and the normal immune Trol controls at both the temperature. The stability of the dry reagents at 37°C was comparable with the stability at the ambient temperature.
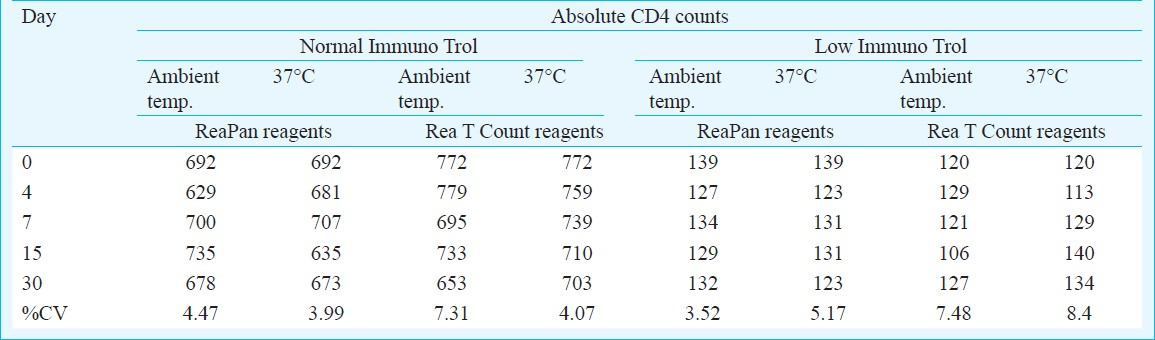
The stability of the samples stained with both Rea T Count and ReaPan dry reagents was assessed at ambient temperature as well as at 37°C. The samples stained with Rea T Count reagents were stable till day 10 at ambient temperature as well as at 37°C. At ambient temperature, the mean %CV for absolute CD4 count was 5.3 (range 4.2-7.0) and at 37°C the mean %CV was 4.2 (range: 1.9-9.1). Whereas the samples stained with the ReaPan reagents, were stable till 7 days at ambient temperature only. (Absolute CD4 count: mean %CV=6.04, range: 2.69-10.7).
Discussion
This study was undertaken to validate the ReaPan and Rea T Count dry reagents against the MultiTEST liquid reagents with TruCOUNT tubes and FACSCount liquid reagents, respectively for the estimation of CD4+ cell counts in HIV infected persons. The reagents were validated for precision, variability and the stability at both ambient temperature and at 37°C. The stability of blood samples stained with both the reagents at ambient temperature and at 37°C was also assessed. The comparison of the absolute values and percentages of CD4+, CD8+ and CD3+ T cells showed good correlation between the dry reagents and reference liquid reagents using the conventional flow cytometer (FACSCalibur) and dedicated flow cytometer (FACSCount) with slightly higher counts using dry reagents. However this should not affect the monitoring of patients for CD4 counts as long as the dry and reference liquid reagents are not used interchangeably. It is important to highlight that this bias did not influence the categorization of those patients who did not yet require ART (i.e. patients with CD4 count >350 cells/μl). On the other hand, 3 and 1 per cent patients were misclassified as having <350 cells/μl using both the ReaPan and Rea T Count reagents respectively indicating chances of earlier initiation of ART, however, it may not influence the monitoring of success of ART.
The Bland Altman analysis showed good agreement between the test and reference reagents (ReaPan dry reagents with the MultiTEST liquid reagents with TruCOUNT tubes and Rea T Count dry reagents with FACSCount liquid reagents). The agreement indicated that the dry reagents and the liquid reagents yielded comparable results with excellent agreement.
The fluorescence intensity of the ReaPan and Rea T Count reagents was comparable with the MultiTEST liquid reagents with TruCOUNT tubes and FACSCount liquid reagents, respectively. The slightly higher bias shown by ReaPan reagents might be attributed to the technical differences and use of different softwares for analysis. It becomes necessary to use Cell Quest Pro software for analysis instead of MultiSET automated software for the samples stained with ReaPan dry reagents, because the location of the fluorescent absolute counting beads is different in case of the ReaPan reagents, as compared to the preset location of the bead attractor gate on the automated MultiSET software. Hence, the technicians performing the procedure need to be trained in the use of Cell Quest Pro software to get optimum results. It was also evident that the vigorous mixing of the dry reagents and the sample is of utmost importance when ReaPan reagents are used. With this additional technical competence, the ReaPan reagents can be used as an alternative for the conventional liquid reagents.
ReaPan and Rea T Count reagents maintain excellent precision with stabilized blood samples i.e. Immuno-Trol controls with %CV less than 5%. These stabilized blood samples are being used as proficiency samples in External Quality Assessment (EQA) for CD4 counts. The precise estimation of CD4 counts in the stabilized blood samples confirms that the dry reagents can also be used while participating in EQA.
The results of the validation of Rea T Count dry reagents confirmed the findings of Bergeron et al10. According to the manufacturer, the reagents are stable at ambient temperature for six months. The present study establishes the stability of ReaPan and Rea T Count reagents at 37°C for one month highlighting utility of these reagents in areas where the temperature might go up to 37°C. On the other hand, the samples stained with ReaPan reagents were found to be stable only at ambient temperature (20 to 25°C) for 7 days indicating slightly less stability as compared to Rea T Count and should be used cautiously. However, even a stability of 7 and 10 days will allow the laboratories to adopt some flexibility in flowcytometry management and avoid repeat blood collection. The stability of the stained blood sample at ambient and higher temperature has great practical application in the field and programmatic settings in minimizing the need for redrawing of the blood sample from patients.
Both the FACSCalibur and FACSCount are maximally used worldover. The potential for the use of dry heat stable reagents, on the existing infrastructure promises to be a cost-effective approach. Not only are the dry reagents less expensive than currently available liquid reagents for FACSCalibur and FACSCount, the shipment costs would also reduce as the maintenance of the cold chain is not necessary.
Most importantly, there will be no additional equipment costs to be borne.
In conclusion, for CD4 count estimation, both ReaPan and Rea T Count dry reagents can be effectively used as an alternative to MultiTEST and FACSCount reagents on FACSCalibur and FACSCount machines respectively with additional training on Cell Quest Pro software on FACSCalibur. The stability of the dry reagents at 37°C will simplify the management of supply and storage and will facilitate the provision of cost-effective CD4 cell count estimations.
Acknowledgment
The authors thank the volunteers for their participation in the study and the clinical and laboratory staff of the National AIDS Research Institute, Pune.
Conflicts of interests: The authors have declared that no competing interests exist.
References
- UNAIDS report on the global AIDS Epidemic UNAIDS/ JC2417E, UNAIDS. 2012. Available from: http://data.unaids.org/pub/Report/2012/2012_epidemic_update_en.pdf
- [Google Scholar]
- The strategic use of antiretroviral: to help end the HIV Epidemic. Available from: http://www.who.int/hiv/pub
- [Google Scholar]
- Annual Report 2008-2009. Department of AIDS Control, Ministry of Health and Family Welfare, Government of India. 2008-2009. Available from: http://nacoonline.org/upload/Publication/Annual_Report_NACO_2008-09.pdf
- [Google Scholar]
- NACO Annual Report. Available from: http://www.nacoonline.org/upload/Publication/Annual%20Report/NACO_AR_Eng%202011-12.pdf
- [Google Scholar]
- Laboratory Guidelines for enumerating CD4 T Lymphocytes in the context of HIV/AIDS, 2007. Available from: http://www.searo.who.int/LinkFiles/BCT_HLM-392.pdf
- [Google Scholar]
- CD4+ T cell count as a tool to monitor HIV progression & anti-retroviral therapy. Indian J Med Res. 2005;121:539-49.
- [Google Scholar]
- Centers for Disease Control and Prevention. Guidelines for performing single-platform absolute CD41 T-cell determinations with CD45 gating for persons infected with human immunodeficiency virus. Morbid Mortal Wkly Rep. 2003;52:1-13.
- [Google Scholar]
- Absolute CD4 T-cell counting in resource-poor settings: direct volumetric measurements versus bead-based clinical flow cytometry instruments. J Acquir Immune Defic Syndr. 2005;39:32-7.
- [Google Scholar]
- Affordable diagnostics - changing the paradigm in India. Cytometry B Clin Cytom. 2008;74(Suppl 1):S117-22.
- [Google Scholar]
- Evaluation of a dry format reagent alternative for CD4 T-cell enumeration for the FACSCount system: A report on a Moroccan-Canadian study. Cytometry B Clin Cytom. 2010;78:188-93.
- [Google Scholar]
- Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-10.
- [Google Scholar]
- Method agreement of quantitative measurements - stability of butanol extracts of resazurin as a Model. Slov Vet Res. 2005;42:77-82.
- [Google Scholar]
- Multiple method comparison: statistical model using percentage similarity. Cytometry B Clin Cytom. 2003;54:46-53.
- [Google Scholar]
- Evaluation of a universal template for single-platform absolute T-lymphocyte subset enumeration. Cytometry. 2002;50:62-8.
- [Google Scholar]






