Translate this page into:
In vitro maturation, fertilization, embryo development & clinical outcome of human metaphase-I oocytes retrieved from stimulated intracytoplasmic sperm injection cycles
Reprint requests: Dr Cristina Álvarez, Laboratorio FIV. Unidad de Reproducción, Servicio de Obstetricia y Ginecología, Hospital General Universitario de Albacete, C/ Hermanos Falcó s/n 02006 Albacete, Spain e-mail: acristina@sescam.jccm.es, cristina10lleo@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The major cause of fertilisation failure after ICSI is failure of the oocyte to initiate the biochemical processes necessary for activation. This inability could be ascribed to cytoplasmic immaturity of those gametes even if they had reached nuclear maturity. The activation of a mature oocyte is characterised by release from metaphase II (MII) arrest and extrusion of the second polar body, followed by pro-nuclear formation. The aim of this study was to evaluate the fate of in vitro matured (IVM) metaphase I (MI) oocytes subjected to intracytoplasmic sperm injection (ICSI) at different time intervals after extrusion of the first polar body (1PB) in in vitro fertilization (IVF) cycles.
Methods:
A total of 8030 oocytes were collected from 1400 ICSI cycles, 5504 MII at the time of cumulus retrieval. Four hundred eight metaphase II (MII) (27.1%) matured to MII after in vitro culture for 2-26 h and 5389 sibling MII in the moment of oocyte denudation were injected. On the other hand, 49 ICSI cycles containing only MI oocytes at retrieval were injected at three different time intervals after reaching the MII. The intervals were as follows: 2-6 h (n=10), 8-11 h (n=4) and 23-26 h (n=10). Fertilization and development potential were evaluated in both studies.
Results:
Fertilization, embryo cleavage and quality were significantly lower in IVM MI compared to MII at time of denudation. Pregnancy rate was higher in group MII. Pregnancy was achieved in three embryo transfers when ICSI was performed within 2-6 h (group I) and 8-11 h (group II) after PB extrusion. One pregnancy was obtained in group I and a healthy neonate was born.
Interpretation & conclusions:
Immature oocytes from women whose ovaries have been stimulated could be matured, fertilized by ICSI, cleaved in vitro and to give rise to a live birth. However, the developmental competence of embryos derived from immature oocytes is reduced, compared with sibling in vivo matured oocytes. Further, human IVM oocytes need between 2-6h after the 1PB extrusion to complete its maturation.
Keywords
Controlled ovarian hyperstimulated cycle
ICSI timing
in vitro maturation
first polar body
metaphase-I
Since the early years of in vitro fertilization (IVF), it has been known that not all oocytes retrieved after controlled ovarian hyperstimulation (COH) have the same potential for achieving pregnancy. Not only the number of oocytes, but also the maturity of the retrieved oocytes is important for the success of assisted reproductive technologies (ART). Between 5 to 20 per cent12of recovered oocytes in COH are immature, either at metaphase I (MI absence of both a germinal vesicle and a first polar body) or germinal vesicle (GV) stage, in human IVF34. Some of these oocytes have the potential for spontaneous maturation during in vitro culture, and may be used as a source of oocytes for sperm injection in ICSI cycles. In patients undergoing COH, the immature oocytes are usually discarded due to the possibility of abnormal embryonic development, or an increased rate of abortion. However, in patients with an unsynchronized cohort of follicles, where the presence of immature oocytes is frequent after stimulation, as in poor responders and poor-prognosis patients5 the use of immature oocytes for IVF is important in order to increase the number of injectable oocytes at time of ICSI6–8.
Oocyte maturation in vitro is a relatively fast event that can occur shortly after oocyte retrieval9 Given that oocyte denudation is performed a few hours after recovery, the maturational profile might be misleading, because some MI oocytes become mature and some GV-stage oocytes move on to MI before oocyte denudation. It is, therefore, necessary to take into account the timing of denudation after oocyte retrieval when fertilization and ensuing embryo development are evaluated.
Embryos derived from immature or in vitro matured (IVM) oocytes are usually deprioritized for transfer. Occasionally, combined transfers of embryos derived from both in vitro and in vivo matured oocytes are performed if there are not enough embryos from in vivo matured oocytes for an appropriated embryo transfer (ET). At the time, it is difficult to tell whether embryos derived from immature or IVM oocytes contribute to pregnancies or live births7–11. The achievement of complete maturation in the oocyte is crucial for the developmental competence of the resulting embryo. It not only depends on nuclear meiotic progression and DNA remodelling, but is also critically influenced by the quality and maturity of ooplasm and alterations of the plasma membrane system12. Human IVF programmes have indicated that delaying insemination improves fertilization rates, presumably by allowing the completion of cytoplasmic maturation for those oocytes that have not completely matured at the time of retrieval13. The timing of maturational events also appears to be tightly regulated and in crucial for the gradual acquisition of oocyte developmental competence14.
The aim of the present study was to evaluate the fate of immature oocytes obtained from stimulated cycles and its possible clinical application in IVF programme. This was analyzed within the framework of a daily laboratory practice, without changing the routine of ovarian stimulation, oocyte retrieval, ICSI technique and embryo culture.
Material & Methods
Patients, ovarian stimulation, and semen analysis: This study was carried out in Human Reproduction Unit of General University Hospital from Albacete, Spain. The study protocol was approved by the Hospital Ethical Committee of Albacete. This retrospective study comprised 814 patients (mean age 34.6 ± 3.6 yr) undergoing 1400 ICSI cycles between January 2002-December 2008.
Female patients were not pre-selected, but were included only if at least one harvested MI oocyte per cycle was present. Couples with severe male factor infertility were excluded. The male factor was the cause of infertility in 26.3 per cent, endometriosis in 4.7 per cent, polycystic ovarian syndrome (PCOS) in 6.6 per cent, tubal factor in 15.4 per cent, poor responders included patients with day 3 plasma FSH >10 IU/ml 6.6 per cent, idiopathic infertility in 5.4 per cent, others in 13.7 per cent and mixed causes of infertility (female and male infertility) in 21.3 per cent couples.
Ovarian stimulation was carried out using standard protocols as previously described15. Semen samples were collected at time of oocytes retrieval. After liquefaction a simple preparative method, such as swim-up, was used and conventional semen parameters were evaluated. When the oocytes extruded the first polar body (PB) at 20 h after in vitro culture, another partner's semen sample was required.
Oocyte retrieval and ICSI procedure: After visualization of the oocyte-cumulus complex (OCC) in the follicular fluid, each oocyte was maintained at 37°C in culture medium (G-IVF™; Vitrolife, Kungsbacka, Sweden) and proper pH was maintained using 6 per cent CO2 in air through all steps.
At 1-2 h after retrieval, the denudation was performed by a brief exposure to buffered medium (G-MOPS™; Vitrolife, Kungsbacka, Sweden) containing 80 IU/ml hyaluronidase (HYASE™-10x; Vitrolife, Kungsbacka, Sweden). To enhance the enzymatic removal of the cumulus and corona cells, the oocytes were aspirated in and out of a hand-drawn Pasteur pipette with a small inner diameter. The denuded oocytes were rinsed several times and incubated until ICSI was performed.
The denuded oocytes were examined under inverted microscope at x200 magnification to assess the nuclear maturation stage. Matured MII oocytes were identified by the presence of a PB and no discernible germinal vesicle nucleus, and were separated from immature prophase-arrested or GV stage oocyte and MI oocytes that had already undergone the GV breakdown but had not extrude the first PB.
This study was conducted in two parts:
Study 1: Comparison of outcome between IVM MI oocytes and those of sibling matured MII oocytes: A total of 1400 ICSI cycles containing mature and immature oocytes after denudation were included.
The denuded oocytes were classified into two groups:
Group 1 consisted of oocytes that had extruded the first PB at the moment of denudation (MII). Using standard ICSI procedure, these oocytes were injected preferably within 2 to 3 h after retrieval.
Group 2 consisted of MI oocytes at the moment of denudation that reached the MII stage after in vitro culture for 2-26 h in 40 μl droplets of G-IVF medium covered with lightweight paraffin oil (OVOIL; Vitrolife, Kungsbacka, Sweden). These oocytes matured in vitro were cultured further for a minimum of 2 h before to be subjected to ICSI.
The presumptive zygotes were assessed for fertilization 17 to 19 h after the procedures. Only zygotes showing two pronuclei (2PN) and 2PB were considered for embryo culture. The number of oocytes showing 1PN or abnormal fertilization, i.e. appearance of 3PN, was also recorded. Neither type of embryo resulting from 1PN or 3PN oocytes was transferred to the patients.
Clinical pregnancy and abortion rates were obtained from cycles with transferred embryos exclusively derived from MII (Group MII) or MI (Group MI) oocytes.
Study 2: Comparison of outcome between IVM MI oocytes for different time periods: To study the developmental potential of MI oocytes from stimulated cycles, 49 ICSI cycles (from 47 patients) containing only immature oocytes at retrieval between January 2002-December 2008 were analysed retrospectively. The male factor was the cause of infertility in 10.6 per cent, endometriosis in 4.3 per cent, PCOS in 14.9 per cent, tubal factor in 8.5 per cent, poor responders included patients with day 3 plasma FSH >10 IU/ml 14.9 per cent, idiopathic infertility in 2.2 per cent, others in 10.6 per cent and mixed causes of infertility (female and male infertility) in 34.0 per cent couples.
The MI oocytes were cultured further in vitro for a maximum of 26 h and during this time these were monitored for the progression of their meiotic maturation after PB extrusion every 2-3 h. Exact timing of polar body extrusion and injection at different time periods should reveal optimal injection time to improved cycle outcomes. Cycles whose MI oocytes were incapable of IVM or cycles whose MI oocytes extruded the first PB after >26 h of incubation were excluded from the study. The mean age of the women was 34.2 ± 2.9 yr (range in age from 28 to 40 yr).
Oocytes matured in vitro were injected at three different time intervals after reaching the MII arrest stage. The intervals were as follows: (i) 2 to 6 h; (ii) 8 to 11 h; (iii) 23 to 26 h. The embryos resulting from the cleavage of zygotes with 2PN were evaluated after 24 h (Day 2). These embryos were classified as grades A, B, C and D according to size; presence of multinucleation and cytoplasmic morphology of blastomeres; percentage of anucleate fragments; cleavage speed and zona pellucida thickness15. Grade A embryos presented cell of equal size with no multinucleated blastomeres and <10 per cent anucleate fragments; grade B embryos presented cells of equal size with no multinucleated blastomeres and less than 25 per cent of fragments; grade C embryos presented cells of unequal sizes with no multinucleated blastomeres and 26 to 35 per cent of fragments; and grade D embryos presented cells of unequal sizes with multinucleated as well as mononucleated blastomeres and more than 35 per cent of fragments. Top-quality embryos had 4 to 5 cells on day 2, along with nonhomogenous zona pellucida.
Embryo transfer, luteal phase and establishment of pregnancy: Embryos were transferred 44-48 h (D2) after oocyte retrieval. The number of embryos transferred depended on the age of the patient, her infertility history, previous treatments and quality of embryos achieved.
The luteal phase was supported by 600 mg per day of natural progesterone (Progeffik®, Effik, Madrid or Utrogestan®, Seid, Barcelona, Spain), beginning 1 day after oocytes retrieval. Pregnancy was diagnosed by the detection of positive result β-human chorionic gonadotropins (>20 mIU/ml), 15 days after oocyte retrieval. Clinical pregnancy was defined as an intrauterine gestation with positive foetal heartbeat seen by transvaginal ultrasound scan. Two weeks after a positive pregnancy test, clinical pregnancy was established by the presence of a gestational sac with fetal heartbeat.
Statistical analysis of results: Results were expressed as mean (±SD) and percentage after normality was assessed by Kolmogorov-Smirnov test and the homogeneity of variance was assessed by Levene test. One-way analysis (ANOVA) of variance with Bonferroni post-hoc analyses and χ2 test were used to compare differences between groups and proportions, respectively. In cases where features were not normally distributed, the non-parametric Kruskal-Wallis test and Fisher's exact test were used in addition to ANOVA and χ2-test. The calculations were carried out using the SPSS 15.0 statistical package (SPSS, Chicago, IL, USA).
Results
A total of 8030 oocytes were collected from 1400 ICSI cycles. Among these, 5504 (68.5%) were MII (degenerating 15 oocytes after denudation), 1506 (18.8%) MI, and 1020 (12.7%) GV and atresics oocytes at the time of cumulus removal. Of the MI oocytes, 408 (27.1%) matured to MII after an in vitro culture of 2-26 h and these were injected (n=408) (Group 2). Within the same ICSI cycle, 5389 (Group 1) sibling oocytes, mature at the moment of oocyte denudation, were injected. Table I represents the comparison of fertilization and embryonic development after ICSI in the two groups of oocytes. Seventeen hours after microinjection, the proportion of oocytes with 2PN was significantly lower in oocytes matured in vitro (55.7%) than in the oocytes matured in vivo (70.7%) (P<0.001). Abnormal fertilization (1PN+3PN) did not differ among groups. When 2PN oocytes matured in vitro were cultured, cleavage rate and development into good morphology embryos (A and B embryos) were significantly (P<0.05) higher in group 1.
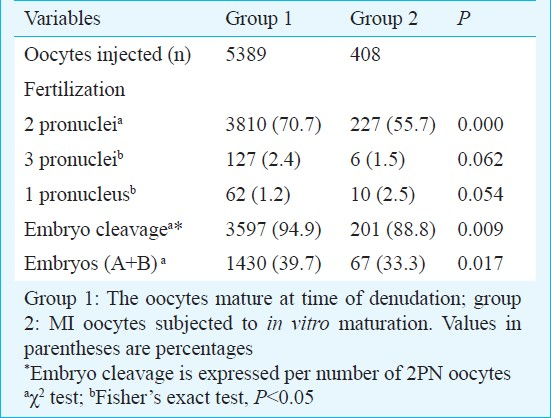
Clinical pregnancy and abortion rates obtained from cycles with transferred embryos exclusively derived from MII (group MII) or MI (group MI) oocytes, are shown in Table II.
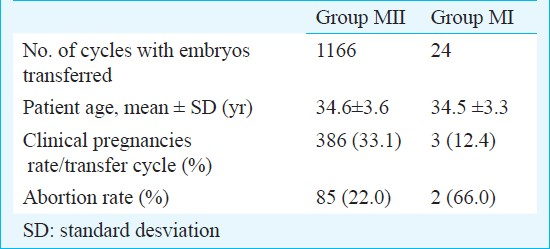
Clinical pregnancy rates were 33.1 per cent in group MII and 12.4 per cent in group MI; and abortion rates were 22 and 66 per cent, respectively.
The study 2 included 49 cycles with a total of 171 oocytes. Among these, 128 (74.9%) were MI oocytes and 43 (25.1%) were GV and degenerated oocytes at time of cumulus removal. Of the MI oocytes, 83 reached the MII stage (30 cycles). ICSI was not performed in 19 cycles because all oocytes remained at the MI stage at the time of ICSI. Complete fertilization failure occurred in 6 cycles. Rates of oocyte maturation, fertilization, cleavage and grade A and grade B embryos transferred are shown in Table III. No significant difference was observed in the fertilization rate among groups. After fertilization, there was significant difference in embryos development between groups II and III, being higher the proportion of embryos development in group III (66.7 versus 100%, P<0.05). However, good-morphology embryos rate (A and B) was significant lower in group III.
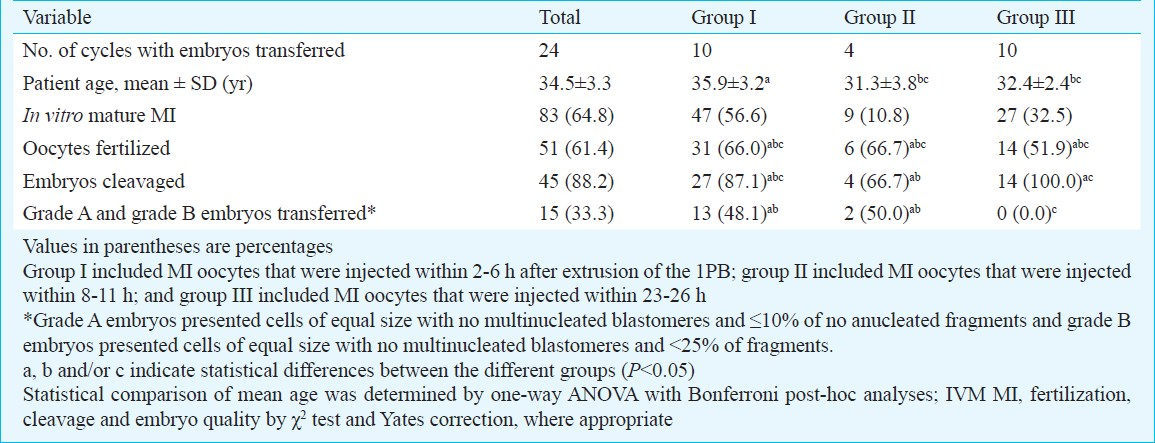
Clinical pregnancy was achieved in three embryo transfers when ICSI was performed within 2-6 h (group I) and 8-11 h (group II) after PB extrusion. One clinical pregnancy was obtained in group I and a healthy neonate was born. The other two clinical pregnancy derived from group II were spontaneously aborted
Different letter indicate statistical differences between groups e.g. patient age is different between group I and II however is similar between groups II and III
Discussion
In the present retrospective study, the immature oocytes collected were from stimulated cycles of patients seeking IVF treatment. Fertilization and embryo cleavage of microinjected IVM MI oocytes were compared to those of sibling MII oocytes. In a stimulated cycle, the administration of gonadotropins creates a supraphysiologic hormonal environment and induces simultaneous growth of small follicles. The heterogeneity of the oocyte population at the time of hCG administration leads to retrieval of oocytes at a different degrees of maturation. It is common for a small percentage of oocytes to arrest at the GV or MI stage. When the percentage of incompetent oocytes is >25 per cent, most of the IVF outcomes are markedly reduced1617. However, the incidence of complete oocyte maturation arrest as a cause of infertility is difficult to estimate.
In an effort to rescue IVF cycles with impaired oocyte maturation, IVM of MI oocytes followed by delayed ICSI was used. The term delayed ICSI is used to describe fertilization by ICSI after IVM of the oocyte. The maturation rates of MI oocytes from previously published reports vary from 16.4-88.3 per cent, depending on period of culture8. In study 1, the maturation rate of MI oocytes after 2-26 h in vitro culture was 27.1 per cent, which is close to the results of Junca et al18 (24.5%) and De Vos et al7 (26.7%), who performed oocyte denudation after 4 h of in vitro culture. Vanhoutte et al19 reported a maturation rate of 41 per cent after 2-4 h of in vitro culture and Shu et al20 obtained 54 per cent matured oocytes after 4-6 h of in vitro culture. Variations in maturation rates might be explained by different culture conditions, addition of growth factors, serum and/or hormones.
In agreement with our results and other reports91920 there is an obvious trend to the effect that reported rates of maturation decrease with an increased time interval between retrieval and oocyte denudation. In some studies where oocyte denudation was performed later, oocytes matured in vitro prior to denudation were counted as oocytes mature at the moment of retrieval. Therefore, the maturational profile might be inaccurate in these situations.
The production of viable oocytes depends firstly on the synthesis and storage of mRNA and protein during oocyte growth and, secondly, on the mobilization and reorganization of these molecules in the final few hours before the oocyte is released from the follicle at ovulation. In vitro matured MI oocytes displayed lower fertilization rates than oocytes already mature at the moment of oocytes denudation (55.7% versus 70.7%, respectively). Our fertilization rates were similar to those obtained in previous reports on ICSI fertilization rates of MI oocyte matured in vitro719 also indicating lower fertilization rates than MII oocytes at moment of oocytes retrieval (42.9 versus 67.4%; 52.7 versus 70.8%). Levran et al21 suggested a correlation between failure of meiotic competence, the developmental features of other follicles in terms of E2 secretion, fertilization and implantation potential of resultant embryos.
Several factors may contribute to incompetent development of immature and IVM oocytes, however, the reduced fertilization rate of IVM oocytes can be explained by the cytoplasmic immaturity of these oocytes in contrast to their nuclear maturation22. The potential of an oocyte to progress through meiosis is acquired only after certain structural and biochemical changes that have occurred in the various compartments of the nucleus and the cytoplasm. Some of the structural changes that occur in the cytoplasm include modifications to the Golgi complex, accumulation of ribosomes, an increase in the number and a change in the morphology of mitochondria and alterations to membrane transport systems in the oocyte. At this stage, one can also notice prominent nuclear changes, these modifications reflect a period of intensive RNA synthesis, which gradually ceases as the oocytes grow. Nuclear maturation involves processes reinitiating meiosis from prophase-I arrested oocytes and driving meiotic division up to the MII stage, at which point meiosis is again arrested until fertilization. Nuclear maturity of oocytes can be evaluated by the presence of the 1PB. Cytoplasmic maturation is less understood and at present no markers are available to evaluate the maturity of the cytoplasm of the oocyte. However, complete cytoplasmic maturation seems to be required for oocyte activation, fertilization and embryonic development, because this is the main factor causing sperm premature chromosome condensation (PCC) after insemination or ICSI in human oocytes23. It has been demonstrated that when insemination of IVM oocytes was delayed for several hours after oocyte retrieval71013, higher proportions of fertilized oocytes developed to advanced preimplantation stages than did the oocytes inseminated immediately after MII arrest. The additional period of maturation needed by oocytes reaching the MII stage in order to be promptly activated by the fertilizing spermatozoa remains unknown.
In previous studies718, fertilized IVM MI oocytes entered normal cleavage, but the present study indicates that IVM MI oocytes, if fertilized, displayed higher rates of cleavage arrest (88.8% versus 94.9%, respectively) than their sibling MII oocytes. Possible explanations for the cleavage arrest include poor culturing conditions, insufficient medium and growth factors, and failure of critical gene expression24.
In this study, clinical pregnancy rate was lower in group MI than group MII, with an higher abortion rate, although the number of clinical pregnancies and abortions were too small to detect any possible significant differences. The exposure of oocytes to artificial conditions of extended in vitro culture seems to be implicated in zona pellucida hardening, resulting in low rate of implantation following IVF. Zona hardening could be other cause involved in the poor outcomes with in vitro mature oocytes2526.
In humans, fertilization rates are enhanced when insemination is delayed for several hours. In our study (study 2), the fertilization rate, proportion of embryos development and good-morphology embryos rate were better when oocytes had matured in culture within 2-6 h. Further, a pregnancy was achieved and a healthy child was born. On the other hand, the oocytes injected between 8-11 h after 1PB extrusion also showed good fertilization, embryo development and embryo quality although two clinical pregnancies spontaneously aborted. When oocytes were injected within 23-26 h after extrusion, 1PB displayed similar fertilization and embryo development rates; however, the quality of embryos was the worst. No pregnancies were obtained. Our results concurred with the earlier results where Li et al27 observed that IVM of MI oocytes for a short period of time might increase the number of available embryos; however, overnight in vitro culture of MI oocytes did not improve results. This may suggest that a minimum of 2-6 h of MII arrest is required to obtain reasonable fertilization rates and embryo quality comparable with those developed from the MII oocytes at the time of retrieval.
Some studies indicate that human oocytes matured in vitro needed at least 1 h incubation after the 1PB extrusion to obtain good fertilization1113. It has been shown that IVM oocytes are sensitive to post-maturation ageing, and delayed sperm injection (after 23-26 h culture) results in a high incidence of pronuclear abnormalities2829. Therefore, defining the optimal duration of MII arrest and the time of ICSI may be essential for the best outcome in human IVM MI. Currently, ICSI has been considered as the best alternative to increase fertilization of IVM oocytes even in conditions where sperm parameters were considered normal, probably due to altered characteristics of the zona pellucida as a result of the longer culture time before insemination30.
In conclusion, the present study showed that immature oocytes from women whose ovaries have been stimulated could be matured, fertilized by ICSI, and cleavaged in vitro to give rise to a live birth. However, the developmental competence of embryos derived from immature oocytes was significantly reduced, compared with sibling in vivo matured oocytes. Even though, the in vitro culture of immature oocytes retrieved from stimulated cycles increases the opportunity for ensuring a pregnancy, patients should be informed of the poor developmental potential of immature oocytes if there are no mature oocytes at the time of oocytes retrieval. On the other hand, human IVM oocytes need between 2-6 h after the 1PB extrusion to complete its maturation.
References
- Maturation in vitro of immature human oocytes for clinical use. Hum Reprod Update. 1998;4:103-20.
- [Google Scholar]
- Oocyte in vitro maturation. In: Gadner DK, ed. Textbook of assisted reproductive technology (2nd ed). London: Martin Dunitz Ltd; 2004.
- [Google Scholar]
- Preincubation of human oocytes may improve fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13:1014-9.
- [Google Scholar]
- Relationship of the human cumulus-free oocyte maturational profile with in vitro outcome parameters after intracytoplasmic sperm injection. J Assist Reprod Genet. 1999;16:483-7.
- [Google Scholar]
- Oocyte in vitro maturation and follicle culture: current clinical achievement and future directions. Hum Reprod. 1999;14(Suppl 1):145-61.
- [Google Scholar]
- Sperm-induced oocyte activation in the rhesus monkey: nuclear and cytoplasmic changes following intracytoplasmic sperm injection. Hum Reprod. 1997;12:1062-8.
- [Google Scholar]
- In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum Reprod. 1999;14:1859-63.
- [Google Scholar]
- Schedule to inject in vitro matured oocytes may increase pregnancy after intracytoplasmic sperm injection. Arch Androl. 2000;44:197-205.
- [Google Scholar]
- The outcome of ICSI of immature MI oocytes and rescued in vitro matured MII oocytes. Hum Reprod. 2004;19:1587-90.
- [Google Scholar]
- Time-dependent capability of human oocytes for activation and pronuclear formation during metaphase II arrest. Hum Reprod. 2004;19:982-7.
- [Google Scholar]
- Optimal ICSI timing after the first polar body extrusion in in vitro matured human oocytes. Hum Reprod. 2007;22:1991-5.
- [Google Scholar]
- Ooplasmic influence on nuclear function during the metaphase II-interphase transition in mouse oocytes. Biol Reprod. 2001;65:1794-9.
- [Google Scholar]
- Effect of delayed insemination on in-vitro fertilization, culture and transfer of human embryos. J Reprod Fertil. 1982;64:285-94.
- [Google Scholar]
- Origins and manifestations of oocyte maturation competencies. Reprod Biomed Online. 2003;6:410-5.
- [Google Scholar]
- Zygote score and status 1 or 2 days after cleavage and assisted reproduction outcome. Int J Gynaecol Obstet. 2008;101:16-20.
- [Google Scholar]
- In vitro maturation for patients with repeated in vitro fertilization failure due to “oocyte maturation abnormalities”. Fertil Steril. 2010;94:496-501.
- [Google Scholar]
- Oocyte maturity and quality: value of intracytoplasmic sperm injection. Fertility of microinjected oocytes after in vitro maturation. Contracept Fertil Sex. 1995;23:463-5.
- [Google Scholar]
- Fertilization, embryo development, and clinical outcome of immature oocytes from stimulated intracytoplasmic sperm injection cycles. Fertil Steril. 2007;87:1022-7.
- [Google Scholar]
- Case Report. Maturation arrest of human oocytes as a cause of infertility. Hum Reprod. 2002;17:1604-9.
- [Google Scholar]
- Nuclear and cytoplasmic maturation of sow oocytes are not synchronized by specific meiotic inhibition with roscovitine during in vitro maturation. Theriogenology. 2005;63:1111-30.
- [Google Scholar]
- Oocyte-genetic aspects. In: Grudzinska JG, Yovich JL, eds. Gemetes. The Oocyte (1st ed). Cambridge: Cambridge University Press; 1995.
- [Google Scholar]
- Zona hardening, zona drilling and assisted hatching: new achievements in assisted reproduction. Cells Tissues Organs. 2000;166:220-7.
- [Google Scholar]
- Do age and extended culture affect the architecture of the zona pellucida of human oocytes and embryos? Zygote. 2006;14:39-44.
- [Google Scholar]
- Evaluation of the developmental potential of metaphase I oocytes from stimulated intracytoplasmic sperm injection cycles. Reprod Fertil Dev. 2011;23:433-7.
- [Google Scholar]
- The chromosomal analysis of human oocytes. An overview of established procedures. Hum Reprod Update. 2005;11:15-32.
- [Google Scholar]
- The cytogenetic constitution of embryos derived from immature (metaphase I) oocytes obtained after ovarian hyperstimulation. Fertil Steril. 2010;94:971-8.
- [Google Scholar]
- Pregnancy and birth after intracytoplasmic sperm injection of in vitro matured germinal-vesicle stage oocytes: case report. Fertil Steril. 1996;65:1047-50.
- [Google Scholar]






