Translate this page into:
Ureaplasma serovars & their antimicrobial susceptibility in patients of infertility & genital tract infections
Reprint requests: Dr Benu Dhawan, Additional Professor, Department of Microbiology, All India Institute of Medical Sciences, Ansari Nagar, New Delhi 110 029, India e-mail: dhawanb@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Ureaplasmas have been implicated in a variety of clinical conditions. However, only certain serovars of ureaplasmas are disease associated. Only a few classes of antimicrobial agents are available for the treatment of mycoplasmal infections in humans. Increase of resistance of genital mycoplasmas to antimicrobials has been reported worldwide. The aim of the present study was to determine the occurrence of Ureaplasma serovars in patients with infertility and genital tract infections with polymerase chain reaction (PCR)–based serotyping. The antimicrobial susceptibilities of Ureaplasma spp. and Mycoplasma hominis were also assessed to determine the most suitable treatment strategy.
Methods:
Sexually active adults (n=147) with symptoms of genital tract infections and 115 infertile women were enrolled. Endocervical swabs from women and urethral swabs from men were subjected to culture and multiplex PCR for detection of genital mycoplasmas. Serotyping of Ureaplasma was done by PCR and antimicrobial susceptibility to doxycycline, azithromycin, josamycin and ofloxacin was done by microbroth dilution method.
Results:
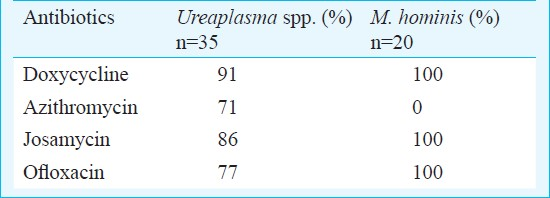
Ureaplasma was detected in 25.8 per cent patients with genital tract infections and 20.8 per cent in infertile women. Serovar 3/14 was the most frequent isolate followed by serovar 1 and serovar 6. The majority of Ureaplasma isolates were susceptible to doxycycline (91%) and josamycin (86%) followed by ofloxacin (77%) and azithromycin (71%). All the isolates of M. hominis were uniformly susceptible to doxycycline, josamycin and ofloxacin.
Interpretation & conclusions:
The predominance of Ureaplasma serovar 3/14 suggests their possible pathogenic role in genital tract infections and infertility. For empirical treatment, doxycycline could be the drug of choice for genital mycoplasmas.
Keywords
Antimicrobial susceptibility
PCR
ureaplasma serovars
Ureaplasmas belong to the normal commensal flora of the genital tract of human beings, with colonisation rates between 60 and 80 per cent worldwide1. However, ureaplasmas are also implicated in invasive diseases such as urethritis, postpartum endometritis, chorioamnionitis, spontaneous abortion and premature birth, as well as low birth weight, pneumonia, bacteremia, meningitis, and chronic lung disease in prematurely born infants2. In studies conducted earlier in India Ureaplasma urealyticum was isolated from 323 and 29 per cent4 of women. In a previous study from our centre, U. urealyticum was recovered from 47 and 45 per cent of men and women with symptoms of genital discharge5. In another Indian study6 U. urealyticum was the predominant organism (56%) isolated from women with chronic cervicitis.
Because of the frequency with which ureaplasmas occur in healthy asymptomatic individuals, it has been suggested that only certain subgroups of the species are pathogenic. The majority of human Ureaplasma isolates belong to U. parvum (biovar 1). U. urealyticum (biovar 2) is isolated less often7. We have also reported U. parvum (biovar 1) as the predominant biovar in patients with genital tract infections8. Ureaplasma consists of 14 serovars. U. parvum includes serotypes 1, 3, 6 and 14, whereas U. urealyticum comprises the remaining 10 serotypes7. Some Ureaplasma serovars have been found to be more frequently associated with clinical diseases; however, the data are limited and conflicting because of the difficulties with traditional genotyping methods9. No studies are available from India on the prevalence of Ureaplasma serovars in clinical diseases.
Mycoplasma infections require the therapeutic use of antimicrobials. Tetracyclines, macrolides and quinolones are the major antibiotics used in the treatment of genital mycoplasmas10. However, their therapeutic efficacy may be unpredictable due to increasing resistance11. The aim of the present study was to determine the distribution of Ureaplasma serovars in patients with infertility and genital tract infections by polymerase chain reaction (PCR) – based serotyping. The antimicrobial susceptibilities of Ureaplasma spp. and M. hominis isolates were also assessed to determine the most suitable strategy for treating these infections.
Material & Methods
All consecutive sexually active adults attending the STD outpatient clinic with symptoms of urethral or cervical discharge, genital pruritis or dysuria and 115 women attending the infertility clinic at the All India Institute of Medical Sciences (AIIMS), New Delhi, India, during January 2010 to December 2010 were included in this study. The medical records of each patient were examined. Patients who had been treated with antibiotics or antifungal agents within the past four weeks were excluded, as were patients who tested positive for Neisseria gonorrhoeae, bacterial vaginosis and Mycobacterium tuberculosis.
A total of 147 patients (59 males, 88 females) with genital tract infections and 115 women with infertility were eligible for enrollment and screened for infection with genital mycoplasmas viz.; Ureaplasma spp and M. hominis. All female patients with genital tract infection were also screened for candidial infection by means of 10% potassium hydroxide (KOH) examination of vaginal secretions.
Ethics Committee approval for study protocol and written informed patient consent were taken for this study.
The specimens included two endocervical swabs from women and two urethral swabs from men. Samples were transported in 2 ml of Pleuropneumonia like organisms medium (PPLO) broth (Difco, USA) containing urea for U. urealyticum and arginine for M. hominis. Serial 10-fold dilutions starting from 1:10 to 1: 105 were prepared. The broths were incubated at 37°C under 5 per cent CO2 and were inspected twice daily for 14 days before discarding as negative. The highest dilution which changed the colour of the indicator present in the broth represented the number of the organisms in the sample in colour changing units per ml (CCU/ml). The reference strains from National Collection of Type Culture Ureaplasma (NCTC10177) and M.hominis (NCTC10111) were used as positive controls.
In addition to culture, multiplex PCR was performed for detection of genital mycoplasmas with primers specific for urease gene of Ureaplasma and 16S rRNA gene of M. hominis to detect the presence of DNA of these two organisms using the protocol by Stellrecht et al12. Briefly, the 50 μl amplification reaction mixture contained 5.00 μl of 10× PCR buffer [1× PCR buffer is 10 mmol/l Tris–HCl (pH 8.8 at 25°C), 50 mmol/l KCl, and 0.1% Triton X-100], 3.0 mM MgCl2, 1.25 U of Taq polymerase (GeneiTaq, Bangalore Genei, India), 400 μmol/l (each) deoxynucleoside triphosphate mixture, 25 pmol of each primer, 16 μl of sample DNA and ultrapure sterile water. The PCR conditions used were initial denaturation at 95°C for 10 min, followed by 35, two-step cycles of 95°C for 15 sec and 60°C for 60 sec, followed by 5 min at 72°C (Fig. I). All the isolates of Ureaplasma were further biotyped in a second PCR targeting the multiple banded antigen (MBA) gene13. The PCR conditions used were initial denaturation at 95°C for 5 min, cyclic denaturation at 94°C for 1 min, annealing at 56°C and elongation at 72°C for 1 min for 35 cycles and final extension at 72°C for 5 min in a thermocycler. PCR positive for biovar 1 were further subtyped into serovars as described earlier7. Briefly, the 50μl amplification reaction mixture contained 10× PCR buffer [1× PCR buffer is 10 mmol/l Tris-HCl (pH 8.8 at 25 °C), 1.5 mM/l MgCl2, 50 mmol/l KCl, and 0.1% Triton X-100], 1 U of Taq polymerase (GeneiTaq, Bangalore Genei, India), 200 μmol each dCTP, dGTP, dATP, dTTP, 10 pmol of each primer, 5 μl of sample DNA and ultrapure sterile water. The PCR conditions used were initial denaturation at 95°C for 5 min, cyclic denaturation at 95°C for 45 sec, annealing at 55°C for 45 sec and elongation at 72°C for 45 sec for 35 cycles and final extension at 72°C for 5 min in a thermocycler. Controls for polymerase inhibition were the DNA for serovar 1, 3/14, and 6 (kindly provided by Dr M.A. De Francisco, Italy). Primers UMS83/UMA269, UMS125/UMA269 and UMS54/UMA269 were used for identification of serovar 1 (398bp), serovar3/14 (442 bp) and serovar 6 (369 bp) respectively (Figs 2–4).
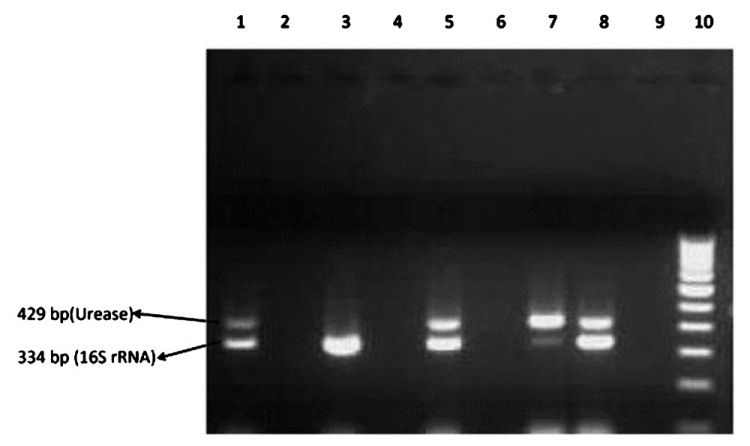
- PCR detection of urease and 16S rNA gene. Lane 1 : U. urealyticum positive control (NCTC 10177; urease positive) and M. hominis positive control (NCTC 10111; 16S rRNA positive; Lane 2, 4 and 9: Clinical samples (negative); Lane 3 : Clinical samples (M. hominis positive); Lane 5, 7 and 8 : Clinical samples (U. urealyticum and M. hominis positive); Lane 6 : Negative control; Lane 10: 100 bp ladder

- Results of PCR amplification for identification of serovar 1; Lane 1: 100 bp Marker; Lane 3, 4 : Negative clinical samples; Lane 2,5-7: Positive clinical samples; Lane 8: Positive control.
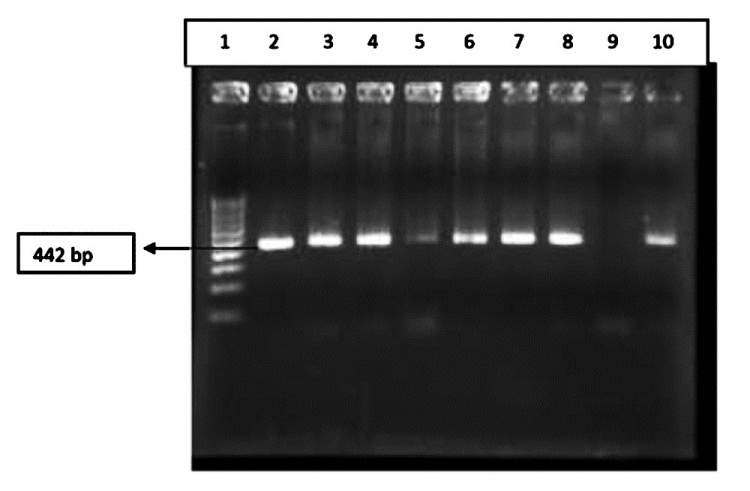
- Results of PCR amplification for MBA gene for identification of serovar 3/14; Lane 1: 100 bp marker; Lane 2: Positive control; Lane 3-8, 10: Clinical samples; Lane 9: Negative control.
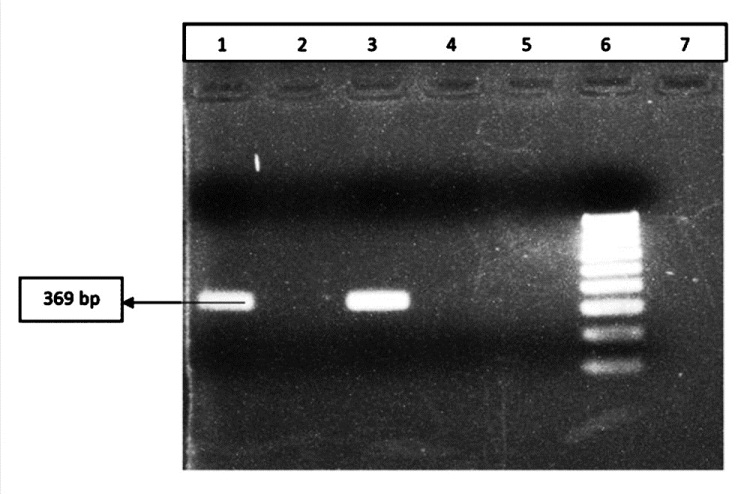
- Results of PCR amplification for identification of serovar 6; Lane 1: Positive control; Lane 2,4: Negative clinical samples; Lane 3: Positive clinical samples; Lane 5 : Negative control; Lane 6: 100 bpMarker.
The isolates of Ureaplasma and M. hominis were subjected to antibiotic susceptibility testing against azithromycin (Hi-Media Laboratories, Mumbai, India), doxycycline (Hi-Media Laboratories, Mumbai, India), ofloxacin (Sigma Aldrich, USA) and josamycin (Alexis Biochemicals, Switzerland) by microbroth dilution method14. Cut-off MICs for susceptibility, intermediate and resistance were based on the values described by Krausse et al15.
Statistical analysis: Pearson Chi-square was used for significance analysis.
Results
Ureaplasma was detected in 38 of 147 [25.8%; 95% confidence interval (CI), 19-34] patients with genital tract infection, of whom 27 of 88 (31%) were females and 11 of 59 (19%) were males and in 24 of 115 (20.8%; 95% CI: 14-29%) infertile women. M. hominis was detected in 18 (12.2%; 95% CI, 7.5-18.6%; 13 females, 5 males) and 8 (6.9%; 95% CI: 3.0-13.2%) patients, respectively. Co-infection with Ureaplasma and M. hominis was detected in five patients (4 females, 1 male) with genital tract infection and one woman with infertility by culture and/or PCR. In addition, microscopic examination showed candidiasis in four (4.5%) of the 88 women with genital tract infection.
Of the 115 infertile women, 53 were cases of primary infertility and 62 of secondary infertility. Ureaplasma was detected in 9 of 53 (17%) and 15 of 62 (24.2%) cases of primary and secondary infertility. M. hominis was detected in three (5.7%) and five (8.1%) cases of primary and secondary infertility, respectively.
U. parvum (biovar 1) was predominant in 38 of 147 (25.8%; 95% CI: 19-34%) patients with genital tract infection and in 24 of 115 (20.8%; 95% CI: 14-29%) infertile women. None of the patients were infected with both biovars. U. parvum isolates were further subtyped into different serovars (Table I). Serovar 3/14 (29/62, 46.8%) was the most frequent isolate in both group of patients followed by serovar 1 (18/62, 29.0%) and serovar 6 (15/62, 24.2%).
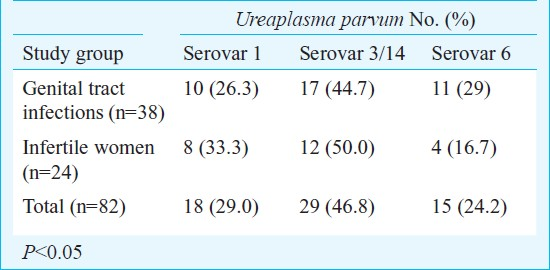
The majority of Ureaplasma isolates were susceptible to doxycycline (91%; 95% CI: 77-98%) and josamycin (86%; 95% CI 69.7-95.2%), 77 per cent were susceptible to ofloxacin (CI: 59.8-89.6%) and 71 per cent to azithromycin (CI: 54-85%) (Table II). Resistance to ofloxacin and azithromycin was of intermediate nature in all the Ureaplasma isolates. The serovars of U. parvum showed no significant difference in their susceptibility patterns to the four antibiotics tested. All the isolates of M. hominis were resistant to azithromycin. No resistance was observed to doxycycline, josamycin and ofloxacin (Table II).
Discussion
Detection of ureaplasmas is possible by characteristic growth on appropriate culture media but species identification of U. parvum and U. urealyticum along with serovar identification by molecular method is important, especially for correct interpretation of laboratory results and evaluation of pathogenicity. U. parvum was found to be predominant isolate in both our study groups as reported by other also9.
Among the different serovars of U. parvum, serovar 3/14 was the most frequent serovar detected in both the study groups. Though the difference in detection rates of the different serovars of U. parvum was not statistically significant, predominance of serovar 3/14 was consistent with previous reports7 and suggests a possible pathogenic role of U. parvum serovar 3/14.
It is possible that the combination of variable serovar specific genes of Ureaplasma with generally known virulence factors determines the development of pathological processes on the mucosal surface of the human genital tract. Further studies are needed to confirm the serovar distribution in different clinical settings and their possible pathogenic role.
Although many different treatment alternatives are available for the treatment of genital mycoplasmas, doxycycline is the most frequently used antibiotic16. However, resistance to tetracycline due to tetM determinant has been observed in both Ureaplasma and M. hominis throughout the world16. Macrolides and especially quinolones have been new treatment alternatives with very high efficacy17. In the present study, doxycycline, azithromycin and ofloxacin were tested as these are the major antibiotics used in the treatment of genital tract infections caused by mycoplasmas10. A further purpose of choosing these antimicrobial agents was because these are conventionally being used for the routine treatment of sexually transmitted infections15. In addition, a new macrolide, josamycin was also tested.
Doxycycline was the most active agent against both Ureaplasma and M. hominis. This finding is consistent with those of other studies conducted in China and Turkey1819. Though doxycycline resistance has been reported in both Ureaplasma and M. hominis18, it was found to be the most active agent against both these pathogens.
Ureaplasma has been considered susceptible to macrolides11. However, in our study Ureaplasma was moderately susceptible to azithromycin. Similar to our findings, azithromycin resistant strains of Ureaplasma are now being reported with increasing frequency1. Resistance to all the isolates of M. hominis to azithromycin was not surprising as M. hominis is known to be intrinsically resistant to macrolide1. No resistance was seen against the new macrolide, josamycin for M. hominis. Our results are in agreement with those reported earlier1119. However, a high rate of resistance to josamycin was observed for Ureaplasma strains. Though resistance to josamycin has been reported for Ureaplasma and M. hominis18, the development of resistance of Ureaplasma isolates in our study was not clear, since the antibiotic is unavailable in our pharmacy and not prescribed in our hospital.
The quinolones are considered useful in the treatment of mycoplasma infection as these are potentially effective against pathogenic species and also including strains resistant to other drugs such as doxycycline20. Although all M. hominis isolates were uniformly susceptible to ofloxacin, it proved to be ineffective against 23 per cent of Ureaplasma isolates. In addition, the doxycycline resistant strains were also resistant to ofloxacin. Similar rates of resistance to ofloxacin has been observed in clinical isolates of Ureaplasma in most of the studies19.
Treatment of mycoplasma infection is imperative to prevent the occurrence of complications. Empirical therapy is important in the treatment of mycoplasmas, since culture and antimicrobial susceptibilities of mycoplasmas are not routinely done in Indian laboratories. Our results indicate that doxycycline should be the first choice drug when empirical treatment is required. Results from previous reports regarding the antimicrobial susceptibilities of genital mycoplasmas, originating from various countries, are controversial1719. Therapeutic failures can be avoided by implementation of empiric treatment regimens based on the determination of antimicrobial susceptibility of genital mycoplasmas in a given geographical area. Thus, it is important to offer the sensitivity test screening periodically and to use drugs on the basis of regularly available sensitivity results.
Acknowledgment
The authors thank the Indian Council of Medical Research, New Delhi for financial support.
References
- Prevalence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women. Int J Infect Dis. 2010;14:90-5.
- [Google Scholar]
- Mycoplasmas and Ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18:757-89.
- [Google Scholar]
- Correlation of mycoplasma with unexplained infertility. Arch Gynecol Obstet. 2009;280:981-5.
- [Google Scholar]
- Correlation of Ureaplasma urealyticum with infertility. Indian J Sex Transm Dis. 1998;19:45-7.
- [Google Scholar]
- Evaluation of the Diagnostic Efficacy of PCR for Ureaplasma urealyticum infection in Indian adults with symptoms of genital discharge. Jpn J Infect Dis. 2006;59:57-8.
- [Google Scholar]
- Detection of Ureaplasma biovar and polymerase chain reaction based-subtyping of Ureaplasma parvum in women with or without symptoms of genital infections. Eur J Microbiol Infect Dis. 2009;28:641-6.
- [Google Scholar]
- Detection and biovar discrimination of Ureaplasma urealyticum in Indian patients with genital tract infections. Diag Microbiol Infect Dis. 2008;60:95-7.
- [Google Scholar]
- Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetrics patients and gynaecological patients with pelvic inflammatory disease. J Clin Microbiol. 1997;35:1199-1202.
- [Google Scholar]
- Susceptibilities of Mycoplasma hominis, M. pneumonia and Ureaplasma urealyticum to GAR-936, dalfopristin, dirithromycin, evernimicin, gatifloxacin, linezolid, moxifloxacin, quinupristin-dalfopristin and telithromycin compared to their susceptibilities to reference macrolides, tetracyclines and quinolones. Antimicrob Agents Chemother. 2001;45:2604-8.
- [Google Scholar]
- Comparison of multiplex PCR assay with culture for detection of genital mycoplasmas. J Clin Microbiol. 2004;42:1528-33.
- [Google Scholar]
- Comparison of PCR, nested PCR and random amplified polymorphic DNA PCR for detection and typing of Ureaplasma urealyticum in specimens from pregnant women. J Clin Microbiol. 1998;36:3032-9.
- [Google Scholar]
- Isenberg H, ed. Clinical microbiology procedures handbook (2nd ed). Washington, D.C: ASM Press; 2004. p. :3.15-1.17.
- In vitro activities of tetracyclines, macrolides, fluoroquinolones and clindamycin against Mycoplasma hominis and Ureaplasma spp. isolated in Germany over 20 years. Clin Microbiol Infect. 2010;16:1649-55.
- [Google Scholar]
- Sexually transmitted diseases treatment guidelines. Morb Mortal Wkly Rep. 2002;51:30-42.
- [Google Scholar]
- Dissemination of the tetM tetracycline resistance determinant to Ureaplasma urealyticum. Antimicrob Agents Chemother. 1986;29:350-2.
- [Google Scholar]
- Comparative in vitro activity of levofloxacin, other fluoroquinolones, doxycycline and erythromycin against Ureaplasma urealyticum and Mycoplasma hominis. J Antimicrob Chemother. 1999;43(Suppl C):33-6.
- [Google Scholar]
- Analysis of detection and antimicrobial resistance of pathogens in prostatic secretion from 1186 infertile men with chronic prostatitis. Zhonghua Nan Ke Xue. 2007;13:628-31.
- [Google Scholar]
- Prevalence and treatment of Chlamydia trachomatis, Ureaplasma urealyticum and Mycoplasma hominis in patients with non-gonococcal urethritis. Jpn J Infect Dis. 2004;57:17-20.
- [Google Scholar]
- Comparative potency of gemifloxacin, new quinolones, tetracycline and clindamycin against Mycoplasma spp. J Antimicrob Chemother. 2000;45(Suppl S1):29-33.
- [Google Scholar]






