Translate this page into:
An amperometric cholesterol biosensor based on epoxy resin membrane bound cholesterol oxidase
Reprint requests: Dr C.S. Pundir, Professor & Head, Department of Biochemistry, M.D. University, Rohtak 124 001, India e-mail: pundircs@rediffmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The use of epoxy resin membrane as a support for immobilization of enzyme has resulted into improved sensitivity and stability of biosensors for uric acid, ascorbic acid and polyphenols. The present work was aimed to prepare an improved amperometric biosensor for determination of serum cholesterol required in the diagnostics and management of certain pathological conditions.
Methods:
Epoxy resin membrane with immobilized cholesterol oxidase was mounted on the cleaned platinum (Pt) electrode with a parafilm to construct a working electrode. This working electrode along with Ag/AgCl as reference and Ag wire as an auxiliary electrode were connected through a three terminal electrometer to construct a cholesterol biosensor.
Results:
The sensor showed optimum response within 25 sec at pH 7.0 and 45°C. The linear working range of biosensor was 1.0 to 8.0 mM cholesterol. Km and Imax for cholesterol were 5.0 mM and 9.09 μA, respectively. The biosensor measured serum cholesterol. The minimum detection limit of the sensor was 1.0 mM. The mean analytical recoveries of added cholesterol in serum (2.84 and 4.13 mM) were 91.4±2.8 and 92.3±3.1 per cent (n=6), respectively. Within and between assay coefficient of variation (CV) were <2 and <4 per cent, respectively. Biosensor had a storage life of 6 months at 4°C.
Interpretation & conclusions:
The use of epoxy resin membrane as a support for immobilization of cholesterol oxidase has resulted into an improved amperometric cholesterol biosensor. The present biosensor had an advantage over the existing biosensors as it worked at comparatively lower potential.
Keywords
Cholesterol
cholesterol biosensor
cholesterol oxidase
epoxy resin membrane
serum
Cholesterol determination is important for the diagnosis and management of hypercholesterolemia, associated with pathological conditions like atherosclerosis, cardiovascular diseases, coronary artery diseases and transient ischaemic heart attacks1. Among the various methods available for determination of cholesterol level in serum such as chemical/colorimetric method, spectrophotometery, thin layer chromatography, gas-liquid chromatography, high-performance liquid chromatography, fluorimetric method, polarographic method and gas chromatography/mass spectrometry (GC/MS), biosensing methods employing immobilized enzymes are the most simple, rapid, specific and sensitive methods. In these biosensors, cholesterol esterase, cholesterol oxidase and peroxidase have been immobilized onto octyl-agarose gel2, poly (2-hydroxyethyl methacrylate) (p(HEMA))/ polypyrrole) membrane3, prussian-blue (PB)4, multiwalled carbon nanotubes (MWCNTs)5, porous silicon6, electrochemically prepared polyaniline film7, amino-undecanethione self assembled monolayer8, cellulose acetate (CA) membrane9, polyvinyl chloride (PVC) reaction cell10, a screen printed electrodes based on carbon nanotubes and cytochrome P45011, dithiobisuccinimidyl propionate self assembled monolayer12, Pt incorporated fullerenes like ZnO nanosphere13, poly (pyrrole) vs poly (3,4-ethylenedioxythiophene) film14, polyaniline/multiwalled carbon nanotubes15 and pencil graphite16. All these supports (organic and inorganic) had one or more disadvantages such as low stability to microbial attack, high cost of the support material or the immobilization procedure, leaching of immobilized enzymes, reduced substrate + enzyme complex formation due to steric hindrance, requirement of high working potential, long response time, metabolite interference and low storage stability10. Epoxy resin membrane, a highly cross-linked network, a long-lived, water permeable membrane has high affinity for enzyme, low cost, easy synthesis, high temperature stability, chemical resistance and porous in nature and thus an ideal material for constructing an enzyme electrode. The application of epoxy resin membrane as a support for immobilization of enzyme has resulted into improved sensitivity and stability of biosensors for uric acid17, ascorbic acid18 and polyphenols19 in our laboratory. It is expected that the use of this membrane would lead to the improved sensitivity and stability of the cholesterol biosensor also. Hence, the present study was aimed to construct an amperometric cholesterol biosensor employing epoxy resin membrane bound cholesterol oxidase.
Material & Methods
The work was carried out in Biochemistry Research Laboratory at M.D. University, Rohtak (Haryana), India.
Sources of chemicals: 4- Aminophenazone from Sigma, USA, cholesterol oxidase from Streptomyces sp., cholesterol, alumina oxide (60-325MESH BSS) and triton X- 100 from SRL, Mumbai, were used. Epoxy resin “Araldite” was purchased from the local market. The kit of enzymic colorimetric method for cholesterol determination was from Erba Transasia, Daman, India. All other chemicals were of analytical grade.
Preparation of enzyme solution: Commercial choles-terol oxidase from Streptomyces sp. (2 mg, containing 26 U) was dissolved in 1.7 ml 0.02 M sodium phosphate buffer, pH 7.0, and stored at 4°C until use. Cholesterol (50 mg) was dissolved in 1.0 ml of triton X 100 by slowly heating and stirring until solution was clear. Sodium phosphate buffer (0.05 M, pH- 7.0) was added to it to get final concentration of 500 mg/dl. Solutions of different concentrations ranging from 50 to 500 mg/dl were prepared and stored at 4°C.
Assay of free cholesterol oxidase20: Reaction mixture consisting of 1.8 ml sodium phosphate buffer (0.05 M, pH 7.0) and 0.1 ml cholesterol oxidase was pre-incubated at 37°C for 2 min. The reaction was started by adding 0.1 ml cholesterol solution (500 mg/dl). After incubating at 37°C for 10 min, 1.0 ml colour reagent was added and kept at room temperature for 15 min to develop the colour and absorbance was read at 520 nm (A520) against control in Spectronic-20D (Thermo, USA) and content of H2O2 generated in reaction was calculated from standard curve between H 2O 2concentration and A520. The soluble protein in dissolved enzyme was determined by Lowry method21.
Fabrication of amperometric cholesterol biosensor
Immobilization of cholesterol oxidase on epoxy resin membrane: The epoxy resin and hardener of ‘Araldite’ were mixed on a plastic (polythene) piece (size 4 × 4 cm) in 85:15 ratio at room temperature for 5 min17. Two ml dissolved cholesterol oxidase was added to this mixture and spread equally to polymerize and cross-linked for 48 h. ‘Araldite’ membrane with entrapped cholesterol oxidase was stripped off the plastic piece and washed with sodium phosphate buffer (0.02 M, pH 7.0). The activity of immobilized enzyme was measured as that for free enzyme except that free enzyme was replaced by epoxy resin membrane bound enzyme and reaction buffer volume was increased by 0.1 ml. The images of epoxy resin membrane with and without enzyme were taken by scanning electron microscopy (SEM) at All India Institute of Medical Sciences, New Delhi.
Fourier Transform - Infra Red (FT-IR) analysis: A drop of mixture of epoxy resin and its hardener was placed between two potassium bromide (KBr) disks for the mid-IR characterization, using a spectrometer (mode iS10, Thermoelectron, USA). For characterization of epoxy groups, the sample was molten between two plain KBr plates separated by a 0.2 mm thick spacer.
Preparation of working electrode and assembling of cholesterol biosensor: The surface of a Pt wire (1.5 × 0.05 cm) was washed with a mixture of 3 per cent hydrogen peroxide and concentrated sulphuric acid in 1:3 ratio for 2 min and washed with distilled water several times. The electrode was polished with 5 μM alumina oxide (diameter 0.05 μm) on its surface and washed again with double distilled water. Epoxy resin membrane with immobilized cholesterol oxidase was mounted on the cleaned Pt electrode with a parafilm to construct a working electrode. This working electrode along with reference (Ag/AgCl) and an auxiliary (Ag wire) electrode were connected through a three terminal electrometer (Keithley Japan, Model 6517A/E) to construct a cholesterol biosensor.
Response measurement of cholesterol biosensor: The three electrode system was immersed into 3 ml of sodium phosphate buffer (0.02 M, pH 7.0) in a 10 ml beaker, 0.2 ml cholesterol solution (12.9 mM) was added into the beaker and the electrodes were polarized at different potential and current (μA) was measured in electrometer. The maximum current was observed at +0.5 V and hence in subsequent experiments, a potential of +0.5 V was applied. The current was also measured at varying concentrations of cholesterol. To construct control, epoxy resin membrane (without immobilized enzyme) was mounted on a Pt electrode. The rest of the procedure was same as described above.
Optimization of working conditions of electrode/biosensor: To determine the pH optimum, the pH of reaction buffer was varied in the pH range of 6.0-9.0 using sodium phosphate buffer (0.02 M). To study optimum temperature, the reaction mixture was incubated at different temperatures ranging from 30 to 70°C at an interval of 5°C for response measurement. To study time required for maximum activity of biosensor, electrode response was measured up to 55 sec at an interval of 5 sec. To study effect of cholesterol concentration, the concentration was varied from 0.1 to 12.0 mM. Michaelis-Menten constant (Km) and maximum reaction current (Imax) were calculated from Lineweaver-Burk (LB) plot22. The limit of detection of the present biosensor was calculated as the lowest quantity of substrate, required to give a signal to a background (blank) +3 times SD of blank.
Amperometric determination of serum free cholesterol: Blood samples (1 ml each) from apparently healthy persons (n=40) and those with hypercholesterolaemia and atherosclerosis (n=40) attending OPD of Pt. B.D. Sharma Post Graduate Institute of Medical Sciences, Rohtak hospital were withdrawn and centrifuged at 1500×g for 10 min at 4°C and their supernatants (serum) were collected. This study protocol was approved by the institute's ethics committee. No sample calculation was done. The samples were collected randomly within the week. The concentration of free cholesterol in serum was determined by the enzyme electrode in the similar manner as described for its response measurement under the optimum working conditions except that the cholesterol solution was replaced by serum. The current (μA) was measured and concentration of cholesterol was extrapolated from the standard curve between cholesterol solution (mg/dl) vs electrical response in μA.
Evaluation of cholesterol biosensor: The biosensor was evaluated by studying its analytic recovery, precision and accuracy/correlation. To determine, accuracy of the sensor, the cholesterol values in 10 serum samples were determined by standard enzymic colorimetric kit method and by the present method and values obtained were correlated using regression equation. The effect of various serum substances was also tested at their physiological concentrations.
Results & Discussion
Immobilization of cholesterol oxidase on epoxy resin membrane: Cholesterol oxidase from Streptomyces sp. was immobilized onto epoxy resin membrane with 88.46 per cent retention of initial activity of free enzyme and a conjugation yield of 0.575 mg/cm2. The -OH groups of epoxy (based on dihydroxydiphenylpropane and epichlorohydrin) containing polymers react with bifunctional polyamine and -NH2 groups of enzyme to form a -C-N- linked enzyme-epoxy amine resin composites. Earlier cholesterol oxidase has been immobilized onto various membranes such as collagen membrane23, nylon membrane24 and cellulose acetate membrane925 for cholesterol biosensor development.
Scanning electron microscopy (SEM): The SEM of epoxy resin membrane without bound enzyme had a uniform polymeric layer, while membrane with bound enzyme had many globular structures (Fig. 1). These observations confirmed immobilization of enzyme.
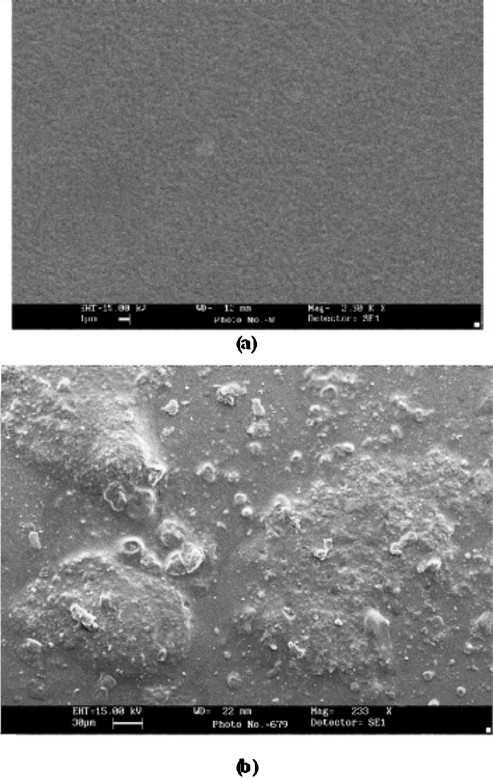
- Scanning electron micrograph (SEM) of cholesterol oxidase bound epoxy resin membrane without enzyme (a) with immobilized cholesterol oxidase (b).
FT-IR spectra of epoxy resin: The IR spectra of epoxy resin revealed the presence of characteristic absorption bands for Ar-C=C-H stretching and bending -CH2 and -CH3 asymmetrical and symmetrical, -C-Ar-O-C stretching, and epoxy CH2-(O- CH-) ring stretching vibration. The presence of epoxy groups in IR spectra was proved from the presence of strong bands at frequencies of 3,056 cm-1 (νC-H epoxy) and 915 cm-1 (γC-O epoxy). The 1, 4-substitution of aromatic ring was seen at 830 cm-1 for epoxy resin. The crosslinking of compound was confirmed by the identification of characteristic absorption peaks. The IR spectrum (Fig. 2) displays a strong broad band in the 3,600-3,200 cm-1 region assigned to O-H stretching vibrations. The appearance of the band at 1,638 cm-1 indicates the formation of OH groups. A strong bands at 1,605, 1,580, 1,510, 1,455 cm-1 are assigned for Ar-C=C-H stretching vibrations. The two bands at 729 and 693 cm-1 may be attributed to out of plan bending of aromatic rings. The disappearance of the bands at 3,056 and 915 cm-1 indicates the opening of epoxy rings. The appearance of the band at 1,109 cm-1 is characteristic for C-N stretching vibrations. The absence of the absorption of epoxy ring and presence of OH group and C-N group confirms the conversion of epoxy group into the corresponding polymer, as well as crosslinking process.
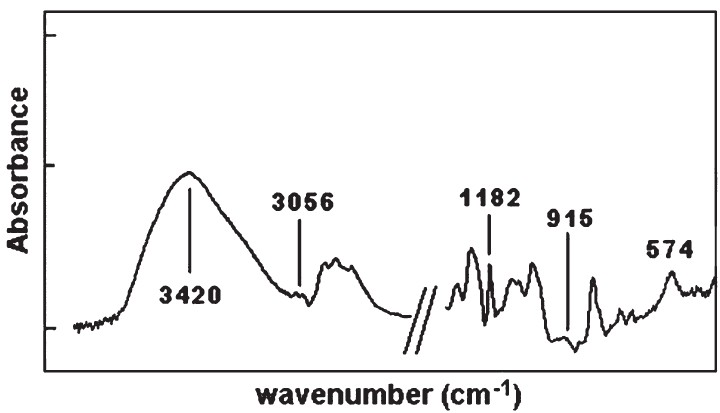
- The partial FT-IR spectra of the crosslinked epoxy resin.
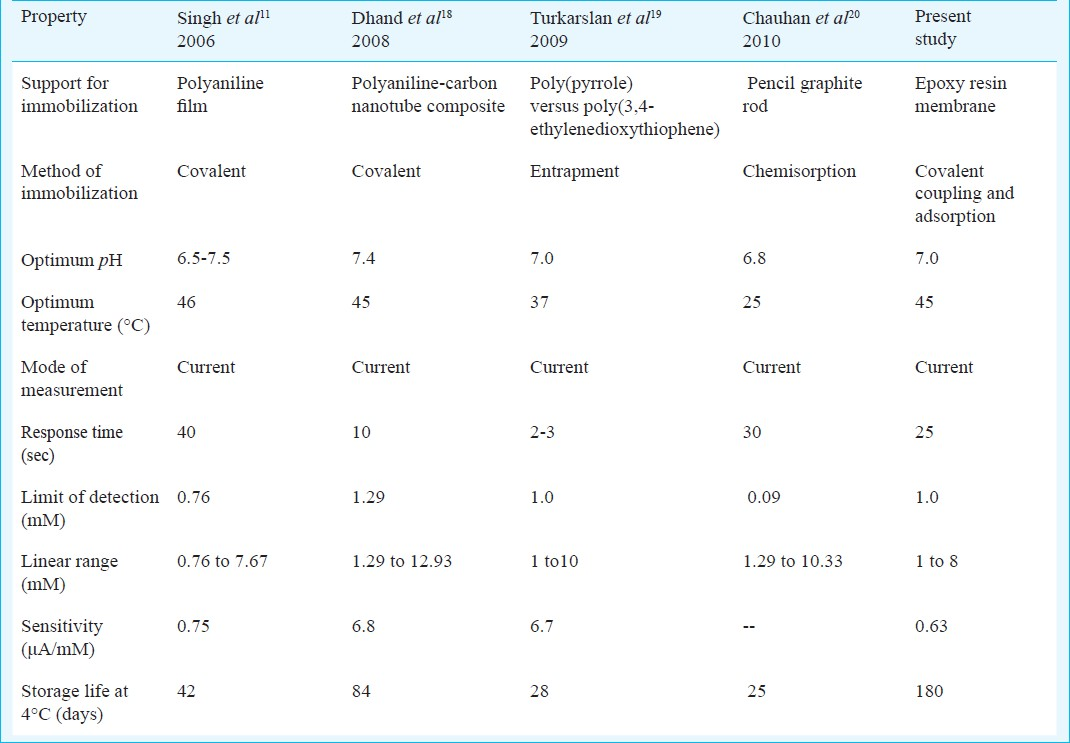
Optimization of biosensor: The sensor showed optimum response within 25 sec at pH 7.0 and 45°C (Fig. 3). At pH 7.0 the epoxy resin membrane bound ChOx must be fully ionized and able to interact with cholesterol. There was a linear relationship between electrode response (current in μA) and cholesterol concentration upto a final concentration of 8.0 mM after which it was constant (Fig. 4). LB plot gave an apparent Km of 5.0 mM and Imax of 9.09 μA (Fig. 4: inset). A comparison of kinetic and analytic property of the present biosensor with those of earlier amperometric cholesterol biosensors is summarized in Table I.
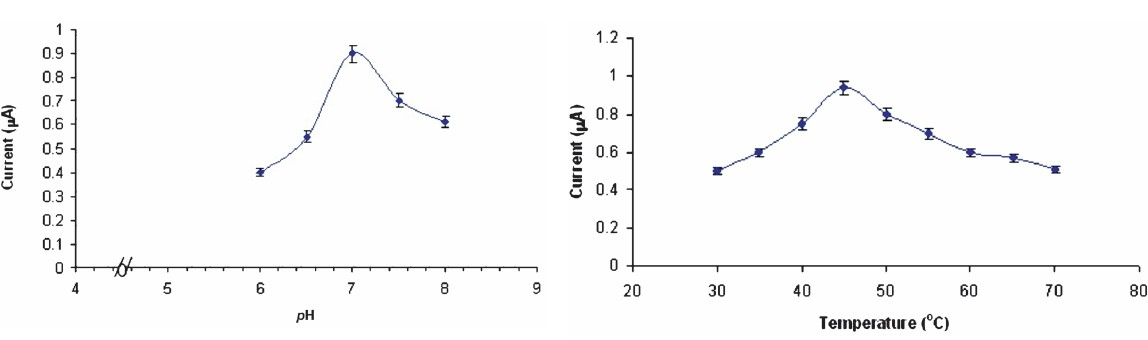
- Effect of pH and incubation temperature on response of cholesterol biosensor based on epoxy resin membrane bound cholesterol oxidase.
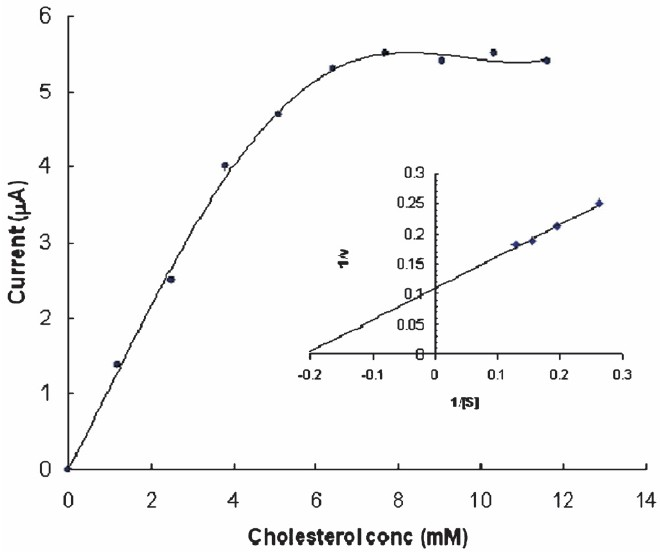
- Effect of cholesterol concentration on cholesterol biosensor response based on epoxy resin membrane bound cholesterol oxidase (inset): Lineweaver-Burk plot of 1/I vs. 1/ cholesterol of epoxy resin membrane cholesterol oxidase.
Determination of serum free cholesterol by biosensor: An amperometric method for analysis of serum cholesterol was developed employing present biosensor. The following parameters were studied in order to evaluate the method:
Linearity: There was a linear relationship between current (μA) and cholesterol concentration ranging from 1 to 8 mM in the reaction mixture, which is comparable to earlier amperometric biosensors, using cholesterol oxidase immobilized onto octyl agarose gel (1-25.8 mM)2, co-immobilized with peroxidase on polyaniline film (1-12.9 mM)3, pencil graphite rod (1.29 to 10.3 mM)16 but higher than those immobilized on carbon paste electrode modified with hydroxymethyl ferrocene and hydrogen peroxide (0.15 mM)6, electropolymerized pyrrole (8 μM)26.
Detection limit: The detection limit of the present amperometric biosensor was 0.1 mM with a sensitivity of 0.63 μA/mM, which is lower than biosensors using cholesterol oxidase co-immobilized with peroxidase on polyaniline (0.64 mM)7, cellulose acetate membrane, (0.32 mM)9, PVC reaction cell (0.11 mM)10 and higher than pencil graphite rod (0.09 mM)16, silica gel bound enzyme (0.003 mM)27 and chitosan-modified glassy carbon electrode (9.5×10-3 mM)28.
Analytical recovery: In order to check the accuracy of the methods, the analytical recovery of added cholesterol in six serum samples of apparently healthy persons was determined. The mean analytical recovery of added cholesterol (2.84 mM and 4.13 mM) in serum was 91.4±2.8 and 92.3±3.1 per cent (n = 6), which is higher than pencil graphite rod (85 and 90%)16 but comparable to electrochemical method employing alkyl amine glass bound enzyme (95-102%) recovery for the added cholesterol at 192 mg/dl and 145 mg/dl29, amperometric method using co-immobilized enzyme using silica gel bound enzyme (95-101% recovery)30 and cellulose acetate membrane (98.86±0.78 & 97.79±0.73%)13 and chitosan-modified glassy carbon electrode (95-101%)28 (Fig. 5).
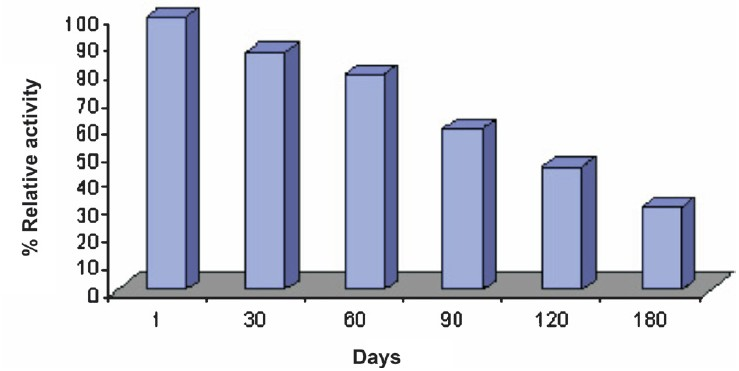
- Effect of storage at 4°C on activity of cholesterol biosensor based on epoxy resin membrane bound cholesterol oxidase.
Precision: To check the reproducibility and reliability of the procedure, the cholesterol content of the sample in one run (within batch) and after storage at -20°C for one week (between batch) were determined. The results showed that the cholesterol value of these determinations agreed with each other and within batch and between batch coefficients of variation (CV) were <2.0 per cent (1.59%) and <2.01 per cent (1.9%) for biosensor. These values are quite close to those reported for other methods such as electrochemical method employing pencil graphite rod (1.59 and 4.15%)16, cellulose acetate membrane (<3 and <5%)9, alkyl amine glass bound enzyme (1.6% for intra-batch and 3.2% for inter-batch)29, amperometric method using silica gel bound enzyme (<1.5% for all samples)30 and chitosan-modified glassy carbon electrode (4.0%)28. Low coefficient of variation indicates that the method is accurate, reproducible and reliable.
Accuracy: In order to know the accuracy of the present method, the values of cholesterol in 10 apparently healthy persons were determined by commercial enzymic colorimetric method (x) and compared with those obtained by present methods (y). The serum cholesterol values obtained by these two methods matched with each other and showed a good correlation (r = 0.991) with the following regression equation: y2= (0.9909x-7.5776). The values obtained by both the methods were also in agreement with the earlier study with pencil graphite rod (r=0.99)16, cellulose acetate membrane (0.990)9 and chitosan-modified glassy carbon electrode (r=0.99)28.
Effect of serum metabolites: To study interference by metabolites, lactate (0.5 mM), glucose (5 mM), uric acid (0.2 mM), ascorbic acid (0.05 mM), acetone (0.17 mM) and bilirubin (5.1 to 17.0 mM) were added to the reaction mixture at their physiological concentrations before addition of cholesterol. Only uric acid and ascorbic acid showed slight increase (12 and 8%) in biosensor response (Table II), while the remaining had no effect. These observations were similar to earlier reports for biosensor having cholesterol oxidase co-immobilized with peroxidase on polyaniline film7. Earlier some interference by uric acid, glucose and lactate for polypyrrole bound enzyme3 and uric acid and ascorbic acid for cellulose acetate membrane bound enzyme has been reported9.
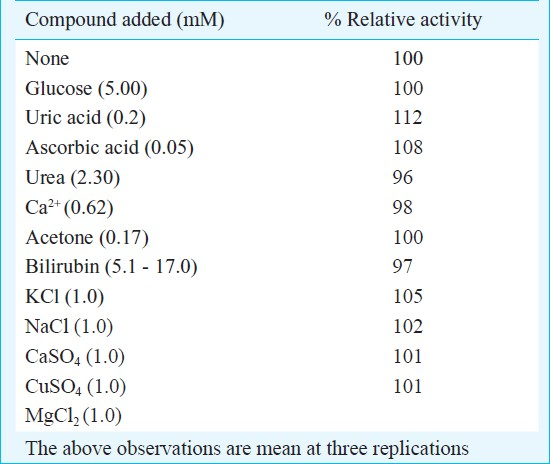
Effect of metal ions: The effect of some metal salts such as KCl, MgCl2, NaCl, CaSO4, and CuSO4, each at a final concentration of 1.0 mM was tested on the response of biosensor. All metals, sodium and potassium ions had practically no effect. These observations were similar to enzyme immobilized on polyaniline film7 and biosensor consisting of co-immobilized enzyme onto cellulose acetate membrane9.
Storage stability and reusability: The enzyme electrode lost 50 per cent of its initial activity after its regular use for 150 times over a period of 6 months, when stored in 0.02 M sodium phosphate buffer, pH 7.0 at 4°C.
In conclusion, a method is described for construction of an amperometric biosensor for estimation of free cholesterol using epoxy resin membrane bound cholesterol oxidase. The biosensor worked efficiently within 25 sec at +0.5V and pH 7.0 and exhibited linearity with cholesterol concentration in the range 1-8 mM and showed temperature tolerance up to 45°C. The sensitivity of biosensor was 0.63 μA/mM with the detection limit of 0.1 mM. The half life of the biosensor was 6 months, when stored in reaction buffer at 4°C. The biosensor had reasonable sensitivity and stability.
Acknowledgment
The authors thank the Head, Department of Biochemistry, Pt. B.D.S. University of Health Science, Rohtak, for providing serum samples. Authors thank the AIIMS, New Delhi for SEM.
References
- Association between metabolic components and subclinical atherosclerosis in Korean adults. Korean J Fam Med. 2012;33:229-36.
- [Google Scholar]
- Amperometric determination of total cholesterol in serum with use of immobilized cholesterol esterase and cholesterol oxidase. Anal Chim Acta. 1982;139:127-32.
- [Google Scholar]
- Amperometric determination of cholesterol in serum using a biosensor of cholesterol oxidase contained within a polypyrrole-hydrogel membrane. Anal Chm Acta. 2001;448:27-36.
- [Google Scholar]
- Amperometric cholesterol biosensors based on the electropolymerization of pyrrole and the electrocatalytic effect of Prussian- Blue layers helped with self- assembled monolayers. Talanta. 2004;64:655-64.
- [Google Scholar]
- An amperometric cholesterol biosensor based on multiwalled carbon nanotubes and organically modified sol- gel/chitosan hybrid composite film. Anal Biochem. 2005;337:111-20.
- [Google Scholar]
- Comparison of effective working electrode area on planar and porous silicon substrates for cholesterol biosensor. Jap J App Phy. 2006;45:7197-202.
- [Google Scholar]
- Covalent immobilization of cholesterol esterase and cholesterol oxidase on polyaniline films for application to cholesterol biosensor. Anal Chim Acta. 2006;568:126-32.
- [Google Scholar]
- Cholesterol biosensor based on covalently immobilized cholesterol oxidase on the Amino-Undecanethiol Self-Assembled Monolayer using Surface Plasma Resonance Technique. Langmuir. 2007;1021:7398-403.
- [Google Scholar]
- Preparation of Pt based amperometric cholesterol biosensor using cellulose acetate membrane. J Scient Indust Res. 2008;67:299-306.
- [Google Scholar]
- Biosensor based on enzyme coupled PVC reaction cell for electrochemical measurement of serum total cholesterol. Sens Actuats B Chem. 2009;136:235-41.
- [Google Scholar]
- Screen-printed electrodes based on carbon nanotubes and cytochrome P450scc for highly-sensitive cholesterol biosensors. Biosens Bioelect. 2008;24:148-50.
- [Google Scholar]
- Dithiobissuccinimidyl propionate self assembled monolayer based cholesterol Biosensor. Analyst. 2007;132:1005-9.
- [Google Scholar]
- Highly sensitive amperometric cholesterol biosensor based on Pt-incorporated fullerene-like ZnO nanospheres. J Phys Chem C. 2010;114:243-50.
- [Google Scholar]
- Poly(pyrrole) versus poly(3,4-ethylenedioxythiophene):amperometric cholesterol biosensor matrices. J Solid State Electrochem. 2009;13:657-63.
- [Google Scholar]
- Polyaniline-carbon nanotube composite film for cholesterol biosensor. Anal Biochem. 2008;83:194-9.
- [Google Scholar]
- Amperometric determination of serum cholesterol with pencil graphite rod. Am J Anal Chem. 2010;2:41-6.
- [Google Scholar]
- Fabrication of dissolved O2 metric uric acid biosensor using uricase epoxy resin biocomposite membrane. Anal Chim Acta. 2009;647:195-201.
- [Google Scholar]
- Construction of an amperometric ascorbate biosensor using epoxy resin membrane bound Lagenaria siceraria fruit ascorbate oxidase. Artif Cells, Blood Sub Biotech. 2010;10:219-23.
- [Google Scholar]
- An amperometric polyphenol biosensor based on laccase immobilized on epoxy resin membrane. Anal Methods. 2011;3:709-14.
- [Google Scholar]
- Fiberoptic cholesterol biocensor with an oxygen optrode as the transducer. Anal Biochem. 1990;184:124-7.
- [Google Scholar]
- Immobilization of cholesterol oxidase on cellulose acetate membrane for free cholesterol biosensor development. Artif Cells Blood Substit Immobil Biotechnol. 2004;32:413-25.
- [Google Scholar]
- Development of a platinized and ferrocene- mediated cholesterol amperometric biosensor based on electropolymerization of polypyrrole in a flow system. Anal Sci. 2002;18:537-41.
- [Google Scholar]
- Purification and properties of a new Brevibacterium sterolicum cholesterol oxidase produced by E. coli MM294/pnH10. FEMS Microb Lett. 2002;25:243-8.
- [Google Scholar]
- Developed a highly sensitive and selective cholesterol biosensor based on direct electron transfer of hemoglobin. Anal Biochem. 2008;383:25-30.
- [Google Scholar]
- Amperometric determination of total cholesterol in serum, with use of immobilized cholesterol ester hydrolase and cholesterol oxidase. Clin Chem. 1977;23:671-6.
- [Google Scholar]
- Automated analysis of total cholesterol is serum using coimmobilized cholesterol ester hydrolase and cholesterol oxidase. J Appl Biochem. 1981;3:84-92.
- [Google Scholar]






