Translate this page into:
Contribution of hepatitis E virus in acute sporadic hepatitis in north western India
Reprint requests: Dr Bharti Malhotra, Associate Professor of Microbiology & Nodal Officer Advanced Research & TB Lab, SMS Medical College, Jaipur 302 004, India e-mail: drbhartimalhotra@gmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Hepatitis E virus (HEV) causes acute viral hepatitis. Majority of the documented studies on hepatitis E have been focused on the incidence of this disease in northern and south central India. Limited data are available on HEV infection among acute sporadic hepatitis cases in north western India. The present study was undertaken to investigate the contribution of hepatitis E virus infection in sporadic hepatitis cases in Rajasthan and neighbouring States.
Methods:
Seven hundred and thirty six patients suspected to have viral hepatitis were screened for the hepatotropic viral markers, hepatitis A, B, C and E by using commercial enzyme immunoassay kits with a high sensitivity and specificity. The acute nature of HEV infection was also confirmed by the detection of HEV RNA by nested RT-PCR.
Results:
Hepatitis E was found to be the major cause of acute sporadic viral hepatitis (49.7%) in this region of India. Mixed infections of HEV-HAV (1.2%), HEV-HBV (6.1%), and HEV-HCV (1.7%) were also detected. No viral marker was detected in 32 per cent cases.
Interpretation & conclusion:
HEV was found as the major aetiological agent of acute sporadic viral hepatitis in Rajasthan (north western India). It is important to screen primarily for all the common enterically and parenterally transmitted hepatotropic viral markers in acute sporadic viral hepatitis. There is a need to do additional serological and molecular tests to identify the aetiological agent in the cases of acute hepatitis.
Keywords
Acute hepatitis
HBV
HCV
HEV
hepatitis
PCR
sporadic viral hepatitis
Hepatitis E was first recognised during an epidemic of hepatitis in Kashmir valley in 19781. It is an enterically transmitted disease that spreads through faecal contamination of drinking water. It occurs both in the form of epidemics as well as sporadic infection in developing countries2–5. According to the South East Asia Regional office of the World Health Organization (WHO), hepatitis E is widespread in developing countries, accounting for up to 90 per cent of all sporadic cases of acute viral hepatitis6. Hepatitis E virus affects young to middle aged adults and causes high mortality in pregnant women, 20-30 per cent as compared to 0.2-1 per cent in general population7. It has been implicated as an important aetiological agent for sporadic fulminant hepatic failure (FHF) in developing countries8.
Aetiology of acute viral hepatitis (AVH) cannot be differentiated on the basis of mode of presentation; confirmation is done serologically. Hepatitis E virus is an important hepatotropic virus that causes acute viral hepatitis. Most of the studies on prevalence of hepatitis E virus are from epidemic setting and some from sporadic setting. Therefore, the present study was undertaken to investigate the aetiology of AVH sporadic cases in Rajasthan and neighbouring States for hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), and hepatitis E virus (HEV). The acute nature of HEV infection was also confirmed by the detection of HEV RNA by nested RT-PCR.
Material & Methods
Patients: From January 2006 to July 2008, serum samples were collected from 736 individuals (aged 10-70 yr) from consecutive adult acute hepatitis cases aged 14-60 yr and only 4 children (aged 10-14 yr) suspected of having viral hepatitis, attending the OPD and wards of the Department of Gastroenterology, Sawai Man Singh (SMS) Hospital, Jaipur, Rajasthan. SMS hospital is a tertiary care centre where patients come from not only Rajasthan but from neighbouring States of Haryana, Punjab, Uttar Pardesh and Gujarat also. The study was approved by the institutional ethics committee and informed written consent was taken from the patients. Patients with signs and symptoms of jaundice, fever, loss of appetite, abdominal pain, scleral icterus, altered sensorium, encephalopathy and fatigue were included in the study. A detailed history of each patient was recorded, including travel history, blood transfusion, food and water intake from outside sources.
Immunoassays: Blood samples were collected from all patients under aseptic conditions and centrifuged at 1200 g. Serum was separated and stored at -80°C for further analysis. The samples were screened using commercially available Micro ELISA for markers of hepatitis A (IgM anti-HAV, Adaltis, Spain), hepatitis B (HBsAg, Biorad Monalisa HBsAg plus, IgM anti HBc in HBsAg positive cases Abbott laboratories North Chicago, IL) hepatitis C (Innotest HCV ab IV, Belgium) and hepatitis E (EIAgen HEV IgM, Adaltis, Spain). On the basis of serological tests, viral hepatitis was classified as acute hepatitis A (presence of IgM anti-HAV), acute hepatitis B (presence of HBsAg & IgM anti HBc), and hepatitis C may be acute or chronic (presence of anti-HCV) or acute hepatitis E (presence of IgM anti HEV), acute hepatitis E and B (presence of anti IgM HEV, HBsAg, IgM anti HBc) and HBV carrier (HBsAg positive). The ELISA kit used for IgM anti HEV were coated with recombinant proteins for open reading frame (ORF) 1 and 2 with 98 per cent sensitivity and specificity. ELISA was performed as per manufacturers’ protocol.
HEV RNA extraction and detection: HEV RNA detection was done in all 356 IgM anti-HEV positive cases and in 28 patients within first five days of illness but negative for all serological markers. Extracted RNA by Guanidinium thiocyanate (GITC) chloroform phenol method with minor modification9 was subjected for cDNA synthesis. cDNA synthesis was carried out using MuLV RT enzyme, reverse primer (20 pmol/ml, Promega) (external anti-sense: 5’- CCG AAT TCA AAG GCA TCC ATG GTG TTT GAG AAT GAC- 3’) RNase out (20 U/μl, Invitrogen, UK), 0.1M DTT and 5 μl templates at 42°C for one hour.
After cDNA synthesis PCR amplification was carried out using the specific previously validated primers selected from non-structural ORF-1 region (Gene Bank accession no. M-32400)10. The primers used were external sense: 5’- CCG GAT CCA CAC ACA TCT GAG CTA CAT TCG TGA GCT- 3’, external anti-sense: 5’- CCG AAT TCA AAG GCA TCC ATG GTG TTT GAG AAT GAC- 3’, internal sense: 5’- GGA ATT CGA CTC CAC CCA GAA TTA CTT- 3’, and internal anti-sense 5’- GGA ATT CAC AGC CGG CGA TCA GGA CAG- 3’. These two sets of primers were designed to produce 343 bp segment of ORF1 region10. The thermal cycling conditions were initial denaturation 94°C for 5 min followed by 30 cycles of denaturation for 30 sec at 94°C, annealing for 30 sec at 59°C and extension for 30 sec at 72°C, as well as final extension for 7 min at 72°C. The final PCR products of 384 patients (356 IgM antiHEV positive cases and 28 negative for all markers but with <5 day illness) were checked on 2 per cent gel electrophoresis stained with ethidium bromide (0.5 μg/ml) under UV transilluminator. For nested PCR, steps were taken to guard against carry over contamination by working in dedicated and physically separated rooms for sample preparation, master mix, amplification and electrophoresis. Each room had dedicated reagents, consumables, filter tips, micro pipettes, furniture, colour coded footwear and gowns, etc. which were not taken out to any other room. Samples were handled in biosafety cabinet. Positive and negative controls were included; sentinel negative controls were also used to check for carry over contamination. The first inner PCR product was handled in a separate safety cabinet from the outer final PCR product.
Results
A total of 736 cases of acute sporadic hepatitis were seen over the study period. HEV was the most common cause followed by HBV, HAV and HCV. Of the 736 patients, 356 (48.3 per cent) patients with acute sporadic hepatitis were positive for IgM anti HEV alone (n=285) or in combination with other virus (n=71) by serology. In almost one third of the patients no viral marker could be detected (Table I). Among 356, 176 had acute viral hepatitis (AVH), 142 had fulminant hepatic failure (FHF) and 38 had subacute hepatic failure (SAHF). There was no mortality in 176 patients of AVH, while 16 (11.27%) FHF patients and 3 (7.8%) SAHF patients died. Of the 142 FHF patients, 24 (16.9%) were positive for HBs Ag, four for HAV IgM.
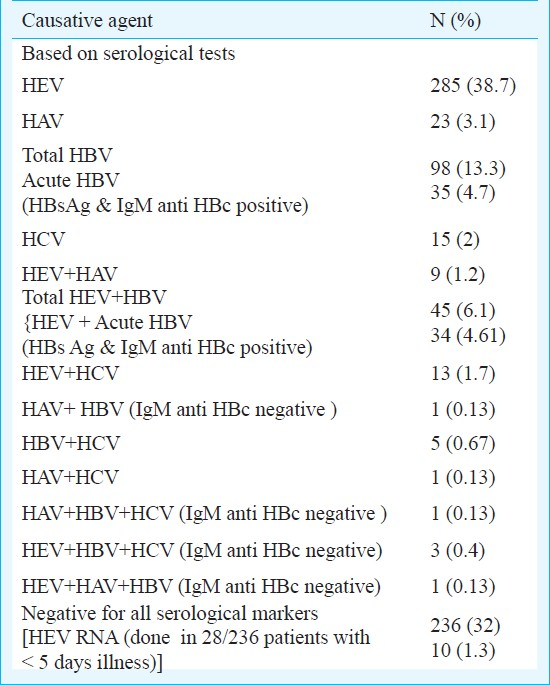
The age of presentation of patients with sporadic acute hepatitis E ranged from 10 to 65 yr (mean ± SD of 32.43 ± 11.07 yr). Maximum numbers of cases of HEV were seen in the age group 15-40 yr. Among 356 IgM anti HEV positive acute sporadic hepatitis E cases, 234 (65.73%) were males and 122 (34.26%) were females. Of the 122 consecutive females, 49 (40.16%) were pregnant. The mortality rate in pregnant females was 20.41 per cent (10/49) and in non-pregnant females was 4.10 per cent (3/73). 17 (34.69%) pregnancies ended in abortion or still birth and in 22 (44.89%) females pregnancy continued unaffected by AVH or normal delivery occurred during the disease course.
All 736 patients had abnormal liver function tests (LFT) suggestive of acute hepatitis; the tests were repeated at weekly intervals. The average duration of abnormal LFT was 11 to 15 days after onset of illness.
Serum samples from 356 IgM anti-HEV positive and 28 of 236 (11.86%) patients of acute sporadic hepatitis (within five days of illness and negative for all serological markers) were investigated for presence of HEV-RNA to confirm acute nature of HEV infection. Of the 356 IgM anti-HEV positives, 223 (62.64 %) and 10 out of 28 (35%) were found to be positive for HEV-RNA. Overall, 366 of 736 (49.72%) patients were found to be HEV positive by either ELISA or PCR.
Discussion
Hepatitis E virus is a major hepatotropic virus for acute viral hepatitis. Hepatitis E exists as sporadic hepatitis with periodic resurgence. This infection is responsible for 30-70 per cent cases of acute sporadic hepatitis11 and is the major cause of acute liver failure (ALF)12. It has been widely reported that HEV primarily affects young adults between 15-40 yr of age in endemic region13. Similar findings were observed in the present study.
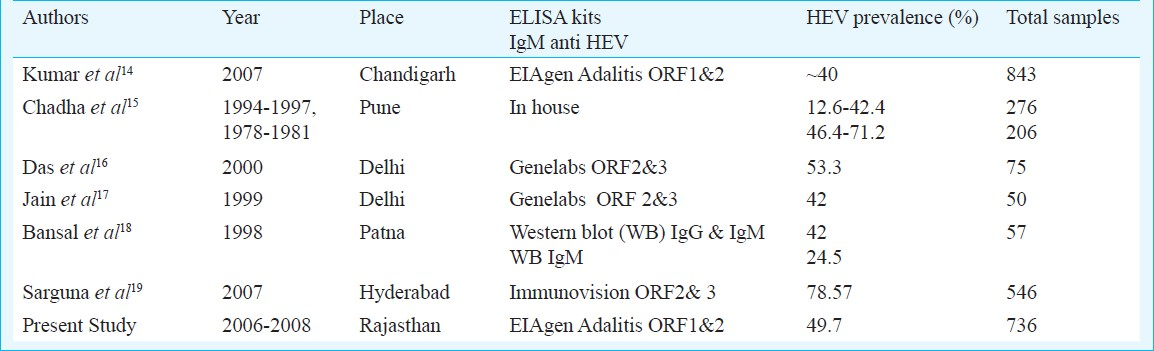
In the present study, among the 736 serum samples obtained from acute sporadic hepatitis patients with jaundice, 366 (49.7%) were positive for HEV alone or in combination with other hepatitis viruses and responsible for about half of the acute viral hepatitis. HEV positivity ranging from 12.6-78.6 per cent has been reported by others from different parts of India (Table II)14–19. Positivity depends on sensitivity and specificity of kits used, population studied, type of samples whether sporadic or from outbreaks. Wide variation has been reported from 17-100 per cent in sensitivity of ELISA kits which depends on recombinant HEV antigens or synthetic peptides used in the kit corresponding to HEV epitopes, kits with ORF 2 sequence have shown little variation in geographically diverse strains in comparison to kits targeting ORF3. Variation may also occur with manufacturers lot and antibodies to different epitopes may differ in persistence20–22. The test may perform differently in endemic and non endemic situation as 33-40 per cent general population has been found to be anti HEV-IgG positive, so difficulty may occur to distinguish between present and past HEV infection2324. Moreover, in immunocompromised individuals antibodies may persist for 6-10 months. Therefore, anti HEV IgM alone may not be informative for diagnosis of acute sporadic HEV infections at times.
In the present study all anti-HEV IgM positive samples were tested for HEV RNA to confirm acute ongoing infection as it has been reported that PCR may be better indicator for acute HEV infection during the first week of illness than ELISA. In our study, 62.64 per cent anti-HEV IgM positive cases were positive for HEV-RNA also. This could be due to the fact that HEV viraemia is transient, occurs in first two wk of infection and declines after 1 wk of onset of Jaundice25. In patients with less than five days history of illness 80.7 per cent were positive for anti-HEV IgM and 85.3 per cent for HEV RNA as observed by us earlier also26. Clayson et al25 observed 93 per cent positivity for HEV RNA and only 79 per cent for IgM anti-HEV while Kumar et al14 reported only 9.4 per cent HEV RNA positivity in AVH cases but 100 per cent positivity in FHF cases in confirmed anti-HEV IgM positive samples. Detection of HEV RNA along with recombinant ORF2 ELISA for anti-HEV IgM could increase positive predictive value for diagnosis of acute HEV infection specially in endemic areas14. However, IgM antibody detection is a better choice for most diagnostic laboratories with serology facilities; HEV RNA testing may also be done in better equipped laboratories within one week of onset of symptoms when other serological tests are negative.
In endemic areas, infection with HEV has been seen in association with other hepatotropic viruses (HAV, HBV and HCV) as also observed in the present study1427–29. Dual infection with HAV, HBV and HCV in acute HEV patients was observed in 1.2, 6.1 and 1.7 per cent cases, respectively, without any squeal. All the HAV and HEV dual infections were possibly co-infections as these have a common route of transmission and all infected patients were less than 14 yr of age. Malathi et al27 observed dual infection of HEV and HAV in 13.4 per cent patients of acute hepatitis in children and Kumar et al28 observed 4.4 per cent patients of acute hepatitis in adults.
In our study, dual acute infection of HEV and HBV was reported in 4.61 per cent and acute HEV on chronic HBV infection was seen in 1.5 per cent of acute hepatitis cases. It could be due to two reasons; one, the population corresponds to meso endemic zone of HBV; second reason could be attributed to the reactivation of latent HBV due to clinical HEV. However, there is little data available to determine such type of infection. In a study from Chennai29, dual infection of HEV and HBV was observed in 5.4 per cent of adults patients of acute hepatitis. Anti-HEV IgM seropositivity rate ranging from 3.2 to 31 per cent in HBsAg carriers has been reported in different studies1430 and hepatitis E has also been suspected to cause super-infection in carriers of hepatitis B31.
The HCV - HEV super-infection was observed in 1.7 per cent cases, all of whom had a history of prior exposure to procedures relating to parenteral route (7 had blood transfusion 4 had surgery and 2 were drug abusers). Studies from Delhi and Chandigarh828 reported dual infection of HEV - HCV in 11 and 7.4 per cent cases, respectively. HCV positivity varies in different geographical areas. Low positivity of HCV has been reported from Rajasthan earlier; 0. 28 per cent by Sood et al32 from a new private hospital and 1.693 per cent by Sharma et al33 from a tertiary care hospital. Mehta et al34 from Jodhpur reported 0.27per cent HEV positivity in blood donors. Since our study was from known AVH cases, 5.1 per cent HCV positivity and 1.7 per cent HEV-HCV positivity are justified as per local trends of HCV infection.
In our study 32 per cent patients were found to be negative for all common hepatotropic viral markers by serological tests, further testing for HEV RNA in 28 samples negative for all serological test in patients with less than five days of illness helped in diagnosing HEV infection in additional patients. However, additional serological and molecular tests should be planned for identifying the aetiological agents in serologically negative cases for HAV, HBV, HCV and HEV to determine whether other viruses like hepatitis G (HGV), Sen Virus, TT virus, etc. exist in this part of India or variant of any other known pathogen.
References
- Study of an epidemic of non-A, non-B hepatitis. Possibility of another human hepatitis virus distinct from post-transfusion non-A, non-B type. Am J Med. 1980;68:818-24.
- [Google Scholar]
- Hepatitis E: epidemiology, aetiology and molecular biology. Rev Med Virol. 1992;2:19-28.
- [Google Scholar]
- Aetiology of acute sporadic non-A, non-B viral hepatitis in India. J Med Virol. 1993;40:121-5.
- [Google Scholar]
- Epidemiology of hepatitis E: Current status. J Gastroenterol Hepatol. 2009;24:1484-93.
- [Google Scholar]
- Etiological role of hepatitis E virus in sporadic fulminant hepatitis. J Med Virol. 1994;42:133-7.
- [Google Scholar]
- Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-9.
- [Google Scholar]
- Enteric non-A, non-B hepatitis: epidemics, animal transmission, and hepatitis E virus detection by the polymerase chain reaction. J Med Virol. 1992;37:263-70.
- [Google Scholar]
- Acute hepatic failure in India: a perspective from the East. J Gastroenterol Hepatol. 2000;15:473-9.
- [Google Scholar]
- Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447-50.
- [Google Scholar]
- The incidence of sporadic viral hepatitis in North India: a preliminary study. Hepatobiliary Pancreat Dis Int. 2007;6:596-9.
- [Google Scholar]
- Comparison of aetiology of sporadic acute and fulminant viral hepatitis in hospitalized patients in Pune, India during 1978-81 and 1994-97. Indian J Gastroenterol. 2003;22:11-5.
- [Google Scholar]
- Role of hepatitis E and other hepatotropic virus in aetiology of sporadic acute viral hepatitis: a hospital based study from urban Delhi. Eur J Epidemiol. 2000;16:937-40.
- [Google Scholar]
- Hepatitis C virus infection in sporadic fulminant viral hepatitis in North India: cause or co-factor. Eur J Gastroenterol Hepatol. 1999;11:1231-7.
- [Google Scholar]
- Outbreak of acute viral hepatitis due to hepatitis E virus in Hyderabad. Indian J Med Microbiol. 2007;25:378-82.
- [Google Scholar]
- Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatitis E Virus Antibody Serum Panel Evaluation Group. Hepatology. 1998;27:857-61.
- [Google Scholar]
- Hepatitis E virus. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, eds. Manual of clinical microbiology (7th ed). Washington, DC: American Society for Microbiology; 1999.
- [Google Scholar]
- Experimental hepatitis E: pathogenesis in cynomolgus macaques (Macaca fascicularis) J Infect Dis. 1993;168:602-9.
- [Google Scholar]
- Diagnostic value of immunoglobulin G (IgG) and IgM anti-hepatitis E virus (HEV) tests based on HEV RNA in an area where hepatitis E is not endemic. J Clin Microbiol. 2000;38:3915-8.
- [Google Scholar]
- Serologic identification of hepatitis E virus infections in epidemic and endemic settings. J Med Virol. 1992;36:246-50.
- [Google Scholar]
- Viremia, fecal shedding, and IgM and IgG responses in patients with hepatitis E. J Infect Dis. 1995;172:927-33.
- [Google Scholar]
- Dynamics of HEV viremia, fecal shedding and its relationship with transaminases and antibody response in patients with sporadic acute hepatitis E. Virol J. 2010;7:213-9.
- [Google Scholar]
- Clinical and viral marker pattern of acute sporadic hepatitis in children in Madras, South India. J Trop Pediatr. 1998;44:275-8.
- [Google Scholar]
- Virological investigation of a hepatitis E epidemic in North India. Singapore Med J. 2006;47:769-73.
- [Google Scholar]
- Serological profile of hepatitis A-E viruses in sporadic acute viral hepatitis in adults in Madras. Indian J Gastroentrol. 1996;15:A116.
- [Google Scholar]
- Seroprevalence of hepatitis B surface antigen, antibodies to the hepatitis C virus, and human immunodeficiency virus in a hospital-based population in Jaipur, Rajasthan. Indian J Commun Med. 2010;35:165-9.
- [Google Scholar]
- Seroprevalence of anti-hepatitis C virus antibody in a hospital-based population of Jaipur, Rajasthan. Indian J Commun Med. 2007;32:158-59.
- [Google Scholar]
- Co-infection rate of HIV and hepatitis C Virus (HCV) among blood donors in Rajasthan, India. Int Conf AIDS. 2002;14 abstract no TuPeA4401
- [Google Scholar]






