Translate this page into:
Paragonimus & paragonimiasis in India
Reprint requests: Dr T. Shantikumar Singh, Professor & Head, Department of Microbiology, Sikkim Manipal Institute of Medical Sciences, Gangtok 737 102, Sikkim, India e-mail: shantikumar_singh@rediffmail.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ever since the discovery of the first indigenous case in 1981, paragonimiasis has gained recognition as a significant food borne parasitic zoonosis in India. The data available on the occurrence of paragonimiasis, until today, may be just the tip of an iceberg as the study areas covered were restricted to Northeast Indian States. Nevertheless, the results of research on paragonimiasis in India have revealed valuable information in epidemiology, life cycle, pathobiology and speciation of Indian Paragonimus. Potamiscus manipurensis, Alcomon superciliosum and Maydelliathelphusa lugubris were identified as the crab hosts of Paragonimus. Paragonimus miyazakii manipurinus n. sub sp., P. hueit’ungensis, P. skrjabini, P. heterotremus, P. compactus, and P. westermani have been described from India. P. heterotremus was found as the causative agent of human paragonimiasis. Ingestion of undercooked crabs and raw crab extract was the major mode of infection. Pulmonary paragonimiasis was the commonest clinical manifestation while pleural effusion and subcutaneous nodules were the common extra-pulmonary forms. Clinico-radiological features of pulmonary paragonimiasis simulated pulmonary tuberculosis. Intradermal test, ELISA and Dot-immunogold filtration assay (DIGFA) were used for diagnosis and epidemiological survey of paragonimiasis. Phylogenitically, Indian Paragonimus species, although nested within the respective clade were distantly related to others within the clade.
Keywords
India
lung fluke
paragonimiasis
Paragonimus heterotremus
tuberculosis
Introduction
Paragonimiasis, also known as endemic haemoptysis, oriental lung fluke infection, pulmonary distomiasis, parasitical haemoptysis, parasitare haemopte, Gregarinosis pulmonum, etc. is one of the most important food-borne parasitic zoonoses caused by one or more of the trematode species of the genus Paragonimus. The disease is endemic in many parts of Asia, Africa and South America1. There are about 50 species of which 11 are known to cause infections in humans, P. westermani has been regarded as the most common and widely distributed human pathogen in Asia2. In South and Southeast Asia, P. heterotremus has been increasingly detected as an important cause of infection in humans3. The natural definitive hosts of the parasite comprise large varieties of wild mammals of the canidae and felidae families and humans. A wide range of fresh water snails, and crabs as well as crayfish served as first and second intermediate hosts, respectively. Humans acquire infection, commonly by ingestion of uncooked or undercooked crustaceans containing metacercariae, the larval stage of the parasite, and rarely by ingestion of infected uncooked or undercooked meat of pig and wild boar, which serve as paratenic hosts45. The parasites primarily infect lung but extra-pulmonary infections are not infrequent. Paragonimiasis is dignosed in the laboratory by microscopic demonstration of Paragonimus ova in the sputum and other clinical specimens such as faeces and pleural fluid or by specific Paragonimus serological tests.
In the past, human paragonimiasis was not considered a problem of public health importance in India until recently. Pulmonary paragonimiasis presenting with bloody sputum or recurrent haemoptysis was generally mistaken for sputum smear negative pulmonary tuberculosis or some other serious conditions with similar symptoms. Research on Paragonimus and paragonimiasis has revealed that paragonimiasis is endemic in many parts of Northeast States of India and provided valuable information on the epidemiology, Paragonimus species, clinico-pathology, diagnosis and treatment. This review is intended to disseminate the research findings and knowledge about Paragonimus and paragonimiasis in India from its confinement in the research laboratories to the clinicians who are directly dealing with the patients and more importantly to raise awareness amid health educators and general population.
Historical review of Paragonimus research in India
Historically, at least two Paragonimus species were first described from India more than a century ago; namely, P. compactus from an Indian mongoose (Herpetes edwardsi) in 18596 and P. westermani from two Bengal tigers in 18787. These mammals were captured in India and died in the zoological gardens in Hamburg in Germany and Amsterdam in Holland, respectively. In 1923, Vevers have described the occurrence of P. compactus and P. westermani in India8. In 1926, Gulati9 had described a new lung fluke species which he named as Paragonimus edwarsi from a palm civet cat, Paradoxurus grayi in Kumaon hills, India, but it was regarded by many as the synonym of P. westermani10. Subsequently, P. westermani infection was described in the lungs of two domestic dogs from Malabar and Coimbatore and a panther from Coorg11 and in a cat from Pantnagar12. About 40 years later Singh and Somvanshi13 reported P. westermani in the lungs of two tigers (Panthera tigris) from the Terai area of the foothills of the Himalayas. The same species was described in the lungs of a beer cat from the zoological park in Chandigarh14. P. westermani was also isolated from the lungs of a mongoose and two tigers which had died of unknown aetiology at the National Corbett Park, India1516 and tigers and cats at Kanha National Park, Mandla (India)1718. In spite of the fact, that paragonimiasis was common among wild mammals and wide spread in India; no record of scientific research on Paragonimus and paragonimiasis in India was available until late 1900s. The earlier reports were mainly focused on autopsy findings of mammalian paragonimiasis. The first epidemiological survey of paragonimiasis in India was conducted in Imphal east district, Manipur19 during the period from 1986 and 1987. In November 1990, the first Indo-Japan joint research on Paragonimus and paragonimiasis was conducted in Manipur to study the life cycle, pathobiology, and morphological characterization of Paragonimus species, and resulted in the discovery of endemic areas of paragonimiasis, identification of crustacean second intermediate hosts and morphological characterization of Paragonimus species occurring in Manipur. Two fresh water crab species namely, Potamiscus manipurensis (Alcock, 1909) and Maydelliathelphusa lugubris (Wood-Mason, 1871)20, formerly known as Barytelpusa lugubris which habitate in most of the mountain streams in Manipur were investigated for Paragonimus metacercarial infection. P. manipurensis was identified as the natural crab hosts harbouring at least three types of Paragonimus metacercariae such as P. heterotremus, P. westermani like metacercariae (large and small types) and easily excysted thin cyst walled metacercariae21. We have described Paragonimus infection in the lungs of a civet cat from Tamenglong district in Manipur22. The morphological features (Carmine stained) of the adult worm isolated from the worm cyst in the lungs were distinct from other species; therefore, it was named as P. miyazakii manipurinus n. sub spp (as a new subspecies) due to close morphological similarity with P. miyazakii. Subsequently P. hueitu’ngensis, P. heterotremus, and P. skrjabini were described from the experimental laboratory animals infected with metacercariae isolated from the crab hosts; Potamiscus manipurensis collected from mountain streams in Manipur23–25. The occurrence of P. heterotremus infection in crab host, B. lugubris (now known as M. lugbris) and human was reported from Arunachal Pradesh26. Recently, an endemic focus of paragonimiasis has been located in Nagaland due to P. heterotremus27. The crab host in Nagaland was found to be P. manipurensis. Tandon et al28 had described the molecular characteristics and ultrastructure of P. westermani metacercariae isolated from M. lugubris in Arunachal Pradesh. Devi et al29 reported the morphological and molecular characterization of P. westermani adult worm isolated from the experimental Wistar rats infected with P. westermani metacercariae harvested from M. lugubris collected from Arunachal Pradesh and Meghalaya States of India and showed that Indian P. westermani complex represented a distinct lineage. In June 2011, we found that fresh water crab species, Alcomon superciliosum (Kemp, 1913) collected from the mountain streams in Moreh town in Manipur (located on the Indo-Myanmar border) were infected with at least two types of Paragonimus metacercariae viz. P. heterotremus and P. westermani (unpublished data).
Paragonimiasis in humans
In India, Paragonimus ova were detected from the sputum and faecal samples from a Chinese patient in Bombay (now Mumbai), Maharashtra in 1919, but believed the infection might have acquired from outside India30. Therefore, this case was not considered as an autochthonous case of paragonimiasis in India. The first indigenous case of human paragonimiasis in India was described by Singh et al31 in 1981in Manipur. The Paragonimus ova were demonstrated in the sputum specimens and identified as ova of P. westermani based on morphological features only. The next case of human paragonimiasis was reported in 1984 from Maharashtra, India32. In 1986, Singh et al33 have described 39 cases of pulmonary paragonimiasis in Manipur. Subsequently, several cases of paragonimiasis were reported from Manipur3435. In an epidemiological survey of paragonimiasis conducted in Imphal east district, Singh et al19 had discovered enedemic areas with an estimated prevalence rate of 6.7 per cent in Manipur. Paragonimiasis cases have been detected almost every year in Manipur but only a few have been published3637. In the Northeast States of India, P. heterotremus has been identified as the causative agent of paragonimiasis in human3839 contrary to the earliest concept that P. westermani was the agent in Manipur.
Life cycle
The parasites utilize two intermediate hosts and complete the life cycle in wild mammals and humans as final definitive hosts.
First intermediate hosts: Fresh water molluscan species of the genera Semisulcospira, Oncomelania, Brotia, and Thiara serve as the first intermediate hosts of Paragonimus species in China, Japan and Thailand2. In India, the molluscan host has not yet been determined. Paragonimus ova discharged either in the faeces and/or sputum of definitive hosts reach water and hatch ciliated miracidia, which swim about to find a suitable snail host. The ova required at least two weeks to complete embryonation and hatching in water40. In the snail host, the miracidium develops into a mother sporocyst which produces asexually first and second generations of rediae, which finally develop into cercariae. The development from miracidium to cercariae in the snail takes about 9 to 13 wk4142. Further development of the cercariae takes place in a suitable crustacean host.
Second intermediate hosts: Fresh water crab species of the genera Potamiscus, Potamon, Paratelphusa, Eriocheir, Geothelphusa, Barytelphusa, and crayfish species of the genus Camberoides and shrimps such as Acrohrachium and Caridina serve as common second intermediate hosts2. In Northeast India, M. lugubris, P. manipurensis and A. superciliosum were found to serve as the crab hosts of Paragonimus. In China a frog species, Rana boulengeri has been described to contain metacercariae of P. skrjabini and served as paratenic host43. The crab hosts are infected with cercariae either by ingestion of the snail hosts or free cercariae directly entering through the soft tissue of the crab host44. A single crab may harbour metacercariae of more than one Paragonimus species simultaneously. The cercariae develop to metacercariae which remained encysted in the liver, gills, intestine, skeleton muscles and sometimes, in the heart of the crab host. For further continuation of the life cycle, the metacercariae are to be taken up by a suitable definitive mammalian host.
Definitive hosts: Many mammals of Canidae and Felidae family and humans serve as definitive hosts. Tigers, civet cats, toddy cats, dogs, mongooses, etc. were found to serve as definitive hosts of Paragonimus species in India. Raccoon dogs, in Japan, served as natural reservoir hosts of P. westermani and P. ohirai4546. In the Philippines and Malaysia, rats serve as natural definitive host as well as paratenic hosts4748. The metacercariae ingested by the definitive hosts excyst larvae in the small intestine, penetrate through the intestinal wall and reach the abdominal cavity in 3 to 6 h49. The larvae migrate into the peritoneal cavity leaving behind haemorrhagic and rusty brown inflammatory exudates and then enter through the diaphragm into the thoracic cavity. Thereafter, the worms wander to find their suitable partners and usually in pairs invade the lung parenchyma to form a worm cyst where they mature to adult worms and start laying ova. A few worms may remain in the pleural cavity or settle in organs and tissues other than the lungs. The time taken by the larval worms to become mature adults varies from one to three months or even longer in some species. Generally, the time taken from infection to laying eggs has been estimated to be from 65 to 90 days50. In their final hosts, the parasites may survive from 1 to 20 years despite the absence of specific therapy.
Species differentiation
Phenotypic characteristics: Traditionally, the study of the morphological characteristics of the adult worms, ova and metacercariae forms the basis of taxonomy of Paragonimus. The important morphological features of adult worms for species differentiation include: (i) shape and size of the whole mount of adult worms, (ii) arrangement of cuticular spines, (iii) relative size of transverse diameters of suckers, (iv) sizes, numbers and branching patterns of ovary and testes, and (v) shape, size and shell character of ova2. The shape, size, number of cysts and presence of pink granules in the body of encysted larvae are important characters for species differentiation of Paragonimus. Most species possess outer and inner cysts whereas some are without cyst, e.g. P. mexicanus. Other useful characters at the metacercarial stage include body proportions of the excysted larvae, relative size of the suckers, the anterior extent of the excretory bladder, the presence and length of oral stylet, the distribution of spines and the number and arrangement of papillae around the suckers51–54.
Genotypic characteristics: Molecular characterization of genomic DNA of parasites can be performed using adult worms, larvae and ova. As the adult worm is not usually recovered in the clinical specimens from patients, molecular characterization of ova is the only option for accurate species identification of the causative agent. A phylogenetic analysis based on the ITS2 region of three species from Manipur and Arunachal Pradesh showed P. westermani from Manipur and Arunachal Pradesh in the same clade as P. westermani and P. siamensis from Thailand (Fig. 1). In addition, P. heterotremus from India were placed close to P. heterotremus from China and Thailand3855. P. skrjabini from Manipur, was closely related to P. skrjabini from China and P. miyazakii from Japan. Blair et al5657 described that P. skrjabini from eastern China (Fujian Province) was phylogenetically very close to P. miyazaki from Japan and proposed that both the populations should be named as the same subspecies as P. skrjabini miyazaki. They also considered P. szechuanensis as the synonym of P. skrjabini and concluded that P. skrjabini represents a separate species complex. Recently, a new lung fluke species, P. pseudoheterotremus morphologically similar to but genetically distinct (different nucleotide sequence in cox1 genes) from P. heterotremus was described from Thailand5859. This finding suggests possible occurrence of cryptic or sibling species to P. heterotremus.
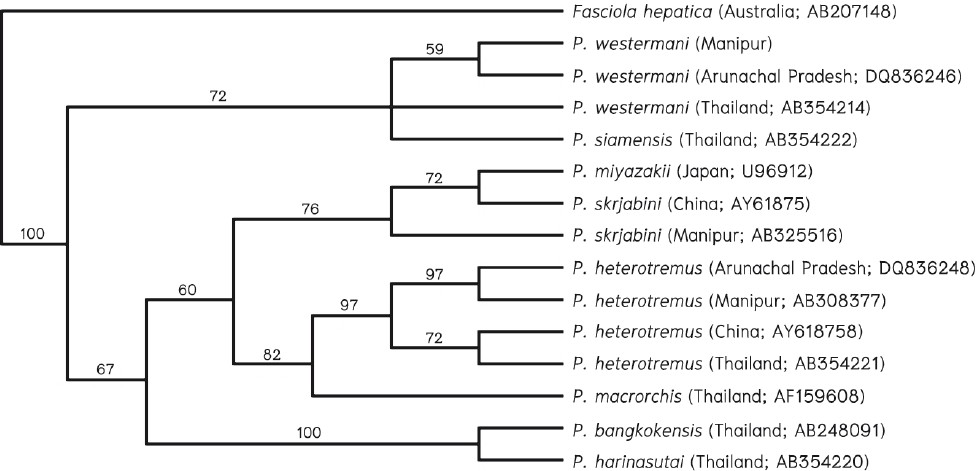
- Phylogenetic analysis of Indian Paragonimus spp. Numbers on the branches are bootstrap values (%) of 1,000 replicates. The sources of data, including geographical origins and GenBank accession numbers are indicated in parentheses. Source: Ref. 37.
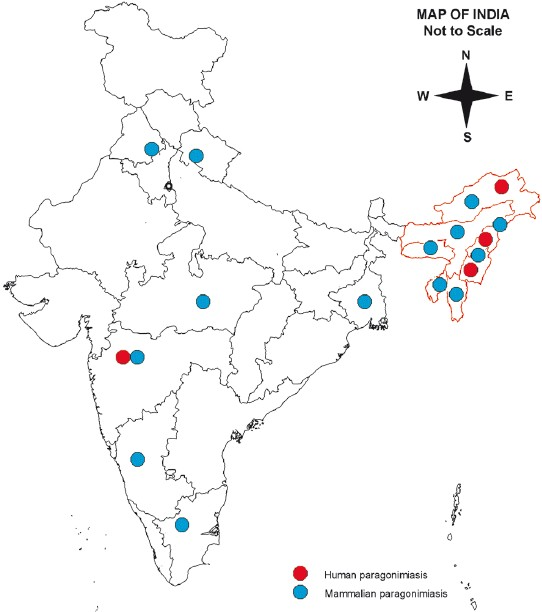
- Geographical distribution of paragonimiasis in India.
Experimental infection of laboratory animals
Experimental infection of laboratory animals is essential to determine the host-parasite relationship between various Paragonimus species and laboratory animals, to study the pathogenesis, to recover adult worms for detailed morphological and molecular characterization, to study immune responses during the course of parasite infection and to obtain antigen for developing immunodiagnostic tests. Therefore, selection of suitable laboratory animal species is a prerequisite for the successful recovery and good yield of the adult parasites. Generally, puppies and kittens are susceptible to all Paragonimus species in Asia and Southeast Asia and served to be the ideal animal model for experimental studies60–63. Puppies were found susceptible to all Paragonimus species occurring in Manipur232464 whereas Wistar rats were found as a suitable rodent model for paragonimiasis in Arunachal Pradesh65.
Pathology
The pathologic lesions of paragonimiasis may be due to invasion and migration by the worms and toxic metabolites and host's immune responses to the invading parasites. Small haemorrhagic and inflammatory spots along the route of migration of the immature worm(s), in the intestine, peritoneum, abdominal organs, diaphragm, thoracic cavity, and pleura, and sometimes, pericardium had been observed in the experimental animals2324. The similar observations were made by other workers and also expected to occur in humans6162. The inflammation may cause adhesion between intra-abdominal organs and peritoneal wall or diaphragm. Sometimes, granuloma may be formed around ova deposited by the migrating adult worms in the abdominal cavity. The basic pathology is the formation of a worm cyst primarily in the lungs. The worm cysts varying from 1 to 3 cm in size usually located superficially, sometimes deep in the lung parenchyma contained dark red or rusty brown viscous fluid, red blood cells, inflammatory cells, eosinophils, necrotic tissue, Charcot-Leyden crystals, and adult worms and ova. The adult worms are usually found at the periphery of the cyst, adhered to the cyst wall. Generally, a cyst contained paired worms, but single or more than two worms of the same or different species may be found5464. The cyst is communicated with bronchioles through which the cystic fluid, including the ova is discharged in the sputum to the exterior environment. If the sputum is swallowed as in case of young children, the ova can be detected in the faeces. Histopathologically, the worm cyst is composed of an outer fibrocollagenous cyst wall with focal haemosiderin-laden macrophages and an inner layer of congested blood vessels, inflammatory cells and normal or distorted ova.
Clinical manifestations
The incubation period may vary from 1 to 2 months or even longer. The shorter incubation period from 2 to 15 days and appearance of early symptoms were observed in 52 per cent of cases in an outbreak of P. westermani infection in Harbin, China66. Clinically, paragonimiasis may be broadly classified into pulmonary, extra-pulmonary and pleuropulmonary forms. Others have categorized paragonimiasis, as acute paragonimiasis, chronic pleuropulmonary paragonimiasis, and ectopic paragonimiasis1.
(i) Pulmonary paragonimiasis: Pulmonary paragonimiasis is the commonest clinical form of paragonimiasis occurring in 76-90 per cent of cases3335. Generally, most patients are ambulatory, apparently healthy and capable of performing normal physical activities. Initially, the patients may present with signs and symptoms of mild pleural effusion, pneumonitis, bronchiectasis or bronchopneumonia. Major presenting symptoms comprised pain or tightness in the chest, difficult breathing, and coughing up rusty brown or blood-stained sputum or recurrent haemoptysis. Haemoptysis may be exacerbated on physical exertion, and massive requiring immediate medical attention and hospitalization. The chronic infection may be associated with fever, anaemia, weakness, and weight loss. Generally, pulmonary infection has high morbidity and low mortality unless complicated with infection in vital organs such as heart, and brain.
(ii) Extra pulmonary paragonimiasis: Extra pulmonary paragonimiasis is due to aberrant migrations of juvenile worms, which deviate from the normal route of migration to the lungs through the intestine, abdominal cavity, diaphragm and pleural cavity. It is likely to occur more commonly in children and in heavy infections. In a review of 45 cases of paragonimiasis in children in Manipur, 44.4 per cent of cases were extra pulmonary35. In another report, extra pulmonary paragonimiasis in adults was seen in 2 per cent of 247 cases67.
Pleural effusion: The migrating worms in the pleural cavity produce pleuritis and effusion; the severity may vary from a minimal to massive effusion depending on the number of worms, frequency of migration and duration. Frank effusion was found in up to 20 per cent cases of pulmonary paragonimiasis in which 60 per cent bilateral, 20 per cent unilateral and 20 per cent encysted were found323519. Uchiyama et al69 reported 5 per cent bilateral and 35 per cent unilateral effusion, 45 per cent nodular or cavity lesion and 15 per cent pleuroparenchymal complex. Persistent effusion was reported in nine patients from Lao PDR69. The main presenting symptoms of patients with pleural effusion were progressive dyspnoea, chest pain and cough with or without haemoptysis.
Cutaneous paragonimiasis: The skin is one of the common sites for ectopic paragonimiasis which may be seen in about 16 per cent of children35. The typical lesion is a migratory subcutaneous nodule which usually appear on the anterior chest wall then migrating through the abdominal wall to the pelvic region or lower limbs without causing much discomfort to the patient except mild pain and itching. The abnormal migration is likely to occur in heavy infection in which the immature worm may invade any organ or tissue except bone. The factors which contribute to abnormal migration are not clearly understood, however, host specificity, and parasite species adaptability may play a major role in the pathogenesis. For example, the Chinese species P. skrjabini is well known to cause extra pulmonary infections in brain, skin, peritoneal cavity and eye66. The cutaneous infection caused by P. skrjabini has been estimated to be between 30 to 60 per cent of the infected patients70. P. skrjabini is also expected to be one of the causative agents of cutaneous paragonimiasis in Manipur. Seven out of 45 cases in childrenin Manipur were found to be cutaneous paragonimiasis; presented with migratory subcutaneous nodules except in one35. Paragonimus adult worm could not be isolated from the excised biopsy materials except in one case who presented with a subcutaneous swelling on the right anterior chest wall. The carmine stained worm was morphologically identified as an adult P. heterotremus71. Generally, the subcuataneous nodule is non tender, mobile, soft to firm in consistency, round to oval measuring 2-5 cm in diameter, and is usually not associated with obvious signs of inflammation or creeping eruption. Rarely, the nodule may remain localized without further migration.The immature or adult worm along with inflammatory exudates, granulomatous tissue, eosinophils, Charcot-Leyden crystals and sometimes Paragonimus ova may be detected in the excision biopsy of the nodule if performed before further migration of the worm.
Cardiovascular paragonimiasis: This is a rare but serious condition which may be associated with pleuropulmonary paragonimiasis. We have described three cases of pleuropulmonary paragonimiasis in children involving heart35. Two of them presented with pericarditis and one child who presented with pericarditis, and myocarditis died of congestive heart failure in spite of praziquantel therapy.
Abdominal paragonimiasis: The symptoms during the prepatent period are usually mild that majority of patients do not recall if they had any gastrointestinal symptoms prior to pulmonary paragonimiasis. Some patients might present with vague abdominal pain, nausea, vomiting, mild diarrhoea and allergic dermatitis. Rarely, patient may present with diarrhoea and dysentery33 and abdominal distension with hepatomegally35.
Cerebral paragonimiasis: Cerebral paragonimiasis has been described as the most common and serious form of extra-pulmonary paragonimiasis, which was reported mainly from China70, Japan72, and Formosa7374. The infection has been described due to erratic migration in which the worms enter the cranial cavity through the jugular or carotid foramen and commonly invading the temporal and occipital lobes75. The condition may be confused with tubercular meningitis or tuberculoma or other space occupying lesions in the brain. The cerebral paragonimiasis is commonly seen in young adults who may present with symptoms of convulsive seizures, especially Jacksonian type, and may be associated with fever, headache, nausea, vomiting, visual disturbance, and motor weakness76. In India, the first case of cerebral paragonimiasis mimicking tuberculoma in a child was reported from Nagaland77. The imaging techniques such as X-ray, CT scans, and MRI are used for specific diagnosis of cerebral paragonimiasis78.
(iii) Pleuropulmonary: The pleuropulmonary paragonimiasis usually occurs when both pleura and lung parenchyma are affected concomitantly. Most patients in the early stage of pleural infection show symptoms of pleurisy and pleural effusion of varying severity followed by parenchymal infection. The clinical manifestations are pain and tightness in the chest, dyspnoea, coughing up blood-stained sputum or haemoptysis and may be associated with fever. The condition was seen in 2.2 per cent of paragonimiasis patients in Manipur33. A high seroprevalence (31.6%) of pleuropulmonary paragonimiasis was also reported from Arunachal Pradesh39.
Laboratory diagnosis
A definitive diagnosis can be made by finding characteristic golden brown, ellipsoidal or oval operculated Paragonimus ova in the clinical specimens such as sputum, aspirated fluids and faeces by microscopy. Occasionally, adult worm may be detected in the excised tissue or cyst or at necropsy or pleural aspirate69 or expectorated in the sputum79. When the ova or worms are not detectable, serological tests play an important but adjunctive role for the diagnosis of extra pulmonary paragonimiasis.
Microscopy
Sputum: Morning sputum samples are ideal for a direct wet smear examination for the parasite ova. The rusty brown or blood-stained sputum usually contains numerous Paragonimus ova and Charcot-Leyden crystals. Earlier studies showed Paragonimus ova in 55.6 to 72 per cent of sputum specimens of pulmonary paragonimiasis cases3380. Devi et al37 found ova positive sputum in 20.9 and 4.1 per cent of pleuropulmonary paragonimiasis in children and adults, respectively. The finding, however, is unusual because sensitivity of sputum examination is expected to be higher in adults than in children.
Pleural fluid: Paragonimus ova may be demonstrated in the centrifuged deposit of pleural fluid in about 10 per cent cases of pleural effusion81. Vidamaly et al69 demonstrated Paragonimus ova in all the pleural aspirats of nine patients with persistent effusion and one of them discharged an adult worm through a draining chest tube. In the absence of demonstrable ova pleural fluid analysis showing glucose content less than 10 mg/dl, lactose dehydrogenase greater than 1000 IU/l, high-protein value, low pH and eosinophilia is indicative of pleuropulmonary paragonimiasis82.
Stool: Stool examination for Paragonimus ova is recommended in children who usually swallow sputum and in patients whose sputum samples are apparently negative for ova. Ideally, two to three stool samples collected at consecutive days should be examined by formolin-ether sedimentation or AMS III concentration technique. The recovery rate varied from 25.6 to 60 per cent, and is higher in children <10 yr of age. Komiya and Yokogawa80 reported Paragonimus ova in 65.1 per cent in the faecal samples of 189 patients of pulmonary paragonimiasis by AMS III concentration technique.
Biopsy: Excision biopsy served as surgical treatment of subcuateous nodule as well as laboratory diagnosis. Adult or immature worm may be found in a carefully dissected nodular or cystic lesion. Microscopic examination of the exudates will show inflammatory cells, eosinophils, Paragonimus ova and Charcot-Leyden crystals. Alternatively, histopathological section will reveal fibrocollagenous tissue, inflammatory cells, eosinophils, sections of the worms and distorted ova.
Immunodiagnosis
The serological tests are important in the diagnosis of extra pulmonary paragonimiasis and to differentiate it from tumours and cysts or nodular lesions caused by other parasites. Some of these tests are used by other researchers for evaluating the therapeutic responses to specific chemotherapy. The various immunological tests which have been evaluated are intradermal (ID) test, complement fixation test (CFT), immunodiffusion, indirect haemagglutination test (IHA), enzyme-linked immunosorbent assay (ELISA), dot-ELISA, and Western blot. ID test is simple, easy to perform, cheap, rapid and highly sensitive and was widely used over the past several years in Japan8384, China85 and in India19 for mass screening and laboratory diagnosis. It is a valuable test in distinguishing pulmonary paragonimiasis from pulmonary tuberculosis, especially in the areas where both are co-endemic. The test is performed by intradermal inoculation of 0.01 to 0.1 ml of the test antigen (saline extract or purified antigenof adult P. westermani) on the volar aspect of the forearm, and the wheal diameters are measured immediately and 15 min after the inoculation. A differential wheal diameter of ⋝5 mm with erythema and pseudopodia indicates a positive test. Exceptionally, a large erythema of ⋝45×35 mm in diameters without an appreciable wheal and pseudopodia may indicate a positive reaction.Whereas a negative skin test almost certainly rules out paragonimiasis, a positive test cannot differentiate between the past and the present infection as the test may remain positive as long as 10 to 20 years even after the successful chemotherapy or spontaneous recovery86. There is also possible cross-reaction with other trematode infections such as schistosomiasis and clornorchiasis, if a crude antigen is used. However, the sensitivity and specificity of the test can be upto100 per cent by using purified antigen87.
CFT has been used in the diagnosis of active infection and to confirm ID positive cases. The test becomes negative within 3 to 9 months after successful treatment87. It was recommended that ID test where used should be applied first in the epidemiological survey and followed by CFT or any other more specific test on individuals who showed positive or doubtful dermal reactions.
Biguet et al87 first developed the immunodiffusion method for the diagnosis of paragonimiasis. Double immunodiffusion technique (Ouchterloney method), immunoelectrophoresis, and counter-current immunoelectrophoresis were reported to be highly sensitive and specific and can be used for speciation by demonstration of specific precipitin bands88–90. IHA is another simple, rapid and sensitive test. In Thailand, the test revealed asensitivity of 88 per cent in the diagnosis of paragonimiasis heterotrema91.
ELISA test for paragonimiasis was first developed by Quicho et al in Thailand92. Since then ELISA based on different techniques and with different antigen preparations have been developed and evaluated for diagnosis of paragonimiasis93–96. The overall specificity of IgG ELISA using the saline extract of adult worms as an antigen was found to be 97 per cent. A 100 per cent sensitivity and specificity could be obtained in an indirect ELISA using F1 antigen fraction to detect antibody against P. heterotremus infection97. The various antigenic components such as 27 kDa possibly excretory/secretary (E/S) products of P. westermani98 and 31.5 kDa substance of P. heterotremus were used as antigens for specific diagnosis of paragonimiasis and for serodiagnosis of human paragonimiasis heterotrema and therapeutic evaluation of praziquantel therapy99, both by ELISA and Western blot, respectively. An enzyme-linked immunoelectrotransfer blot was developed for differential diagnosis between P. heterotremus and P. westermani infections100. Other ELISA techniques are sandwich ELISA using monoclonal antibodies-based antigen detection assay101 and multiple dot-ELISA95 now used in Japan. Generally, ELISA tests are used to detect parasite specific IgG antibodies but the detection of specific IgE antibodies was proven to reduce cross-reactions with other trematode infections and detection of parasite specific IgM antibodies was recommended in the diagnosis of infection at the early stage102103. In India, Regional Medical Research Centre (RMRC, ICMR), Dibrugarh had developed IgG ELISA using E/S antigen for diagnosis of paragonimiasis and was reported to be highly sensitive and specific104. The ELISA tests are now most widely used for serological diagnosis of paragonimiasis due to their high sensitivity and specificity. The tests are also applicable to mass screening. However, ELISA tests are more expensive, time-consuming and require costly equipment and experienced persons and all the reagents, antigens, in particular, are not commercially available.
Rapid test: Recently, dot-immunogold filtration assay (DIGFA) kit was developed in China for anti-P. westermani antibody detection. This is found to be simple and rapid and does not require any special devices and/or experienced technicians and the results are obtained within 10 min. This kit was prepared using P. westermani antigen and reported in China to have the sensitivity and specificity up to 99 and 92 per cent, respectively105.
Haematological investigation
Leucocytosis with relative lymphocytosis, eosinophilia and increased ESR were common findings in patients with paragonimiasis33. Most patients have haemoglobin value within normal limits in spite of frequent spiting of blood and recurrent haemoptysis. Eosinophilia (absolute eosinophil count >1000/μl) and increased ESR upto 104 mm at the end of 1st h (Westergren) were consistently found in children with paragonimiasis in Manipur35.
X-rays and other imaging technique
The common radiographic findings were patchy air-space consolidation or opacity with associated pleural reaction or thickening, cystic or cavitary lesions, pleural effusion (usually bilateral) and nodular opacities, which were difficult to differentiate from the similar lesions of tubercular origin. The chest radiographs showed patchy consolidations in 62-71 per cent, pleural thickening in 28 per cent, cystic or cavitary lesions in 11-14 per cent, effusion in 9-10 per cent, and nodular lesions in 8-13 per cent33–35. Persistent pleural effusion in nine patients was reported from Lao PDR of whom in 44.4 per cent patients showed bilateral effusion69. Compared to the chest X-ray, computerized tomography (CT) was found as a better technique for visualization of the lesions in the lungs106. Burrows and tunnels joining the cystic lesions have been described in broncho-tomogram or pulmonary CT107108. Chest radiographs may be normal in symptomatic patients3132. Higher rate of normal chest roentgenograms in pulmonary paragonimiasis was also reported from endemic areas in Nigeria109. CT and MRI of brain of cerebral paragonimiasis patients will show conglomerates of multiple ring-shaped shadows called the ‘grape cluster’ or ‘soap bubble appearance’110111 or isodense lesion mimicking tuberculoma77 in one hemisphere of the cerebral cortex.
Treatment
Three major antihelminthic drugs are currently available for treatment of paragonimiasis. Praziquantel is the drug of choice for both pulmonary and extra pulmonary paragonimiasis112. The recommended dose is 25 mg/kg body weight administered orally three times a day after meals for three days without any appreciable side effects. With this regimen relapse occurred in about 2 per cent cases. A 100 per cent cure rate was obtained when the therapy was extended up to 5 days34. Bithionol, 2, 2’-thiobis [4,6-dichlorophenol] was the drug used before the praziquantel was available for the treatment of paragonimiasis. It is given in doses of 40 mg/kg body weight in two equally divided doses on alternate days for a course of 10 to 20 doses33. The most common side effect was urticaria, which was observed in about 50 per cent of patients after 2 or 3 doses of bithionol. Rarely, urticaria may be very severe requiring hospitalization and parenteral antihistaminics.The other side effects such as nausea, vomiting, diarrhoea and itching were generally few, mild and transitory. Zhong et al113 had used bithionol to treat paragonimiais in doses of 50 mg/kg body weight per day on alternate days for 20 doses and found a cure rate of 97.1 per cent. Recently, triclabendazole {5-chloro-6 (2,3-dichlorophenoxy)-2methylthio benzimidazole}, a drug used in veterinary medicine was evaluated for the treatment of paragonimiasis in humans. Control trials have shown that triclabendazole when administered in a single dosage of 10 mg/kg body weight had comparable efficacy, safety and tolerability with praziquantel114115.
Conclusion
In the recent years, paragonimiasis has emerged as an important food-borne parasitic disease in India, mainly in the Northeastern States of India. Failure to recognize pulmonary paragonimiasis has resulted in over diagnosis of pulmonary tuberculosis and unwarranted antitubercular therapy, which will have a negative impact on the outcome of the Revised National Tuberculosis Control Programme, especially in the tuberculosis endemic areas. Mahajan emphasized the need to generate awareness among the clinicians and public regarding paragonimiasis and to consider this disease in the differential diagnosis of PTB in places where both co-exist116. P. heterotremus has been identified as the causative agent of human paragonimiasis in the northeast India. Potamiscus manipurensis, M. lugubris and A. superciliosum were identified as second intermediate crab hosts of Paragonimus in these regions. Further research work is required to determine the first intermediate snail hosts of Paragonimus species in India and the role of P. skrjabini and P. westermani in human paragonimiasis. The control strategies for paragonimiasis should include the epidemiological surveys to determine the magnitude of the problem, training of public health care providers about the diagnosis and management of paragonimiasis, screening of all patients attending TB clinics, DOTS microscopy centers, hospitals and rural health centers for both tuberculosis and paragonimiasis. People should be educated not to consume fresh and improperly cooked crabs and crayfish, and to clean hands, utensils, cutlery boards, strainers, knives, etc. after processing fresh crabs and crayfish. Public health authority should ensure the continuous supply of praziquantel in the hospitals and dispensaries. The problem of paragonimiasis is likely to continue in India unless awareness among public and medical practitioners is spread and appropriate control measures taken.
Source of Support: Nil
Conflict of Interest: None declared
References
- North American paragonimiasis (caused by Paragonimus kellicotti) in the context of global praragonimiasis. Clin Microbiol Rev. 2009;22:415-46.
- [Google Scholar]
- Lung flukes in the world: Morphology and life history. In: Symposium on Epidemiology of Parasitic Disease. International Medical Foundation of Japan; 1974. p. :101-34.
- [Google Scholar]
- Paragonimus and paragonimiasis in Vietnam. In: Asian Parasitology 1. Food-borne helminthiasis in Asia. Chiba Japan: Federation of Asian Parasitologists; 2005. p. :149-53.
- [Google Scholar]
- Recent progress in studies in Paragonimus and paragonimiasis control in China. Chinese Med J. 1981;94:483-94.
- [Google Scholar]
- Immature lung flukes first found in the muscles of wild boar in Japan. J Parasitol. 1976;62:836-7.
- [Google Scholar]
- Observations on the genus Paragonimus Braun, 1899 with a redescription of P. compactus (Cobbold, 1859) J Helminth. 1923;1:9-20.
- [Google Scholar]
- On the occurrence of a lung fluke Paragonimus edwardsi, n.sp. in a palm civet (Paradoxurus grayi) in Kumaon hills. Mem Dept Agri India Vet Ser. 1926;3:187-90.
- [Google Scholar]
- Lung flukes in two dogs in Madras Presidency. Indian J Vet Sci Anim Hus. 1935;5:30-2.
- [Google Scholar]
- The occurrence of Paragonimus westermani in the lungs of cats in India. Indian J Vet Sci Anim Hus. 1938;8:255-7.
- [Google Scholar]
- Paragonimus westermani in tigers (Panthera tigris) in India. J wildlife Dis. 1978;14:322-4.
- [Google Scholar]
- Paragonimiasis in a bear cat (Articus binturong) Ann Trop Med Parasitol. 1978;72:391-3.
- [Google Scholar]
- Trematodes of the mongoose Herpestes edwardsii Geofroy from Visakhapatnam District. Proc Indian Acad Sci B. 1979;88:421-4.
- [Google Scholar]
- Helminth parasites from tiger (Panthera tigris) in India. Indian J Parasitol. 1980;4:71-2.
- [Google Scholar]
- Bronchial hyperplasia in a tiger (Panthera tigris) Indian J Anim Sci. 1988;58:230-3.
- [Google Scholar]
- Paragonimus infection in tigers at Kanha National Park. J Parasitol Appl Anim Biol. 1994;3:115-6.
- [Google Scholar]
- Some freshwater crabs from Northeast India bordered on Myanmar. J Teikyo Heisei Univ. 2012;23:199-213.
- [Google Scholar]
- Three types of Paragonimus metacercariae isolated from Potamiscus manipurensis in Manipur. Indian J Med Microbiol. 1997;15:159-62.
- [Google Scholar]
- A case of paragonimiasis in a civet cat with a new Paragonimus subspecies in Manipur, India. Indian J Pathol Microbiol. 1998;41:351-3.
- [Google Scholar]
- Occurrence of the lung fluke Paragonimus hueit’ungensisin Manipur, India. Chinese Med J (Taipei). 2002;65:426-9.
- [Google Scholar]
- Occurrence of the lung fluke Paragonimus heterotremus in Manipur, India. Chinese Med Sci J. 2003;18:20-5.
- [Google Scholar]
- Possible discovery of Chinese lung fluke, Paragonimus skrjabini in Manipur, India. Southeast Asian J Trop Med Public Health. 2006;37(Suppl 3):53-6.
- [Google Scholar]
- Paragonimus and paragonimiasis - A new focus in Arunachal Pradesh, India. Curr Sci. 2003;l84:985-7.
- [Google Scholar]
- Paragonimus heterotremus infection in Nagaland: A new focus of paragonimiasis in India. Indian J Med Microbiol. 2009;27:123-7.
- [Google Scholar]
- Surface fine topography and PCR-based determination of metacercariae of Paragonimus sp.from edible crabs in Arunachal Pradesh, Northeast India. Parasitol Res. 2007;102:21-8.
- [Google Scholar]
- Morphological and molecular characterization of Paragonimus westermani in northeastern India. Acta Tropica. 2010;116:31-8.
- [Google Scholar]
- Surveyer. Case of Distoma Disease in India. Indian J Med Res (special Indian Sci congress). 1919;1:214-6.
- [Google Scholar]
- Pulmonary paragonimiasis: Clinical features, diagnosis and treatment of 39 cases in Manipur. Trans R Soc Trop Med Hyg. 1986;80:967-71.
- [Google Scholar]
- Pleuropulmonary paragonimiasis mimicking pulmonary tuberculosis-a report of three cases. Indian J Med Microbiol. 2005;23:131-4.
- [Google Scholar]
- Morphological and molecular characterizations of Paragonimus heterotremus, the causative agent of human paragonmiasis in India. Southeast Asian J Trop Med Public Health. 2007;38(Suppl 1):82-6.
- [Google Scholar]
- Pleuropulmonary paragonimiasis due to Paragonimus heterotremus: molecular diagnosis, prevalence of infection and clinicoradiological features in an endemic area of northeastern India. Trans R Soc Trop Med Hyg. 2007;101:786-92.
- [Google Scholar]
- Pragonimiasis: An important food-borne zoonosis in China. Trends Parasitol. 2008;24:318-23.
- [Google Scholar]
- Experimental completion of the life cycle of the lung fluke, Paragonimus westermani in the laboratory. Jpn J Parasitol. 1981;30:173-7.
- [Google Scholar]
- Experimental infection of the fresh water crab, Geothelphusa dehaani, with the cercariae of Paragonimus miyazakii. Jpn J Parasitol. 1986;35:183-9.
- [Google Scholar]
- Rana boulengeri as the paratenic host of Paragonimus skrjabini. Chinese J Parasitol Parasitic Dis. 1985;3:157-9.
- [Google Scholar]
- The route of infection of Paragonimus westermani (diploid type) cercariae in the fresh water crab, Geothelphusa dehaani. J Helminthol. 1991;65:38-42.
- [Google Scholar]
- A case of multiple lung fluke (Paragonimus westermani) infection of a raccoon dog in Japan. Jpn J Parasitol. 1985;34:513-6.
- [Google Scholar]
- An epidemiological survey of the lung fluke, Paragonimus spp. in wild mammals in northern part of Hyogo Prefecture, Japan. Jpn J Vet Sci. 1985;47:911-9.
- [Google Scholar]
- Paragonimus westermani filipinus Miyazaki, 1978, stat. n. occurring at Jaro, Leyte, the Philipinus. Med Bull Fukuoka Univ. 1979;6:447-62.
- [Google Scholar]
- Growth of Paragonimus westermani (Kerbert, 1878) in mammals and mode of transmission of the fluke among mammals. Jpn J Trop Med Hyg. 1996;24:225-32.
- [Google Scholar]
- Morphological description and life cycle of Paragonimus spp.(Trematoda: Troglotrematidae): causal agent of human paragonimiasis in Colombia. J Parasitol. 2003;89:749-55.
- [Google Scholar]
- Paragonimiasis. In: LI YL, Guan XH, eds. Human parasitology. People's Public Health Press; 2005. p. :109-13.
- [Google Scholar]
- The occurrence of a new type of Paragonimus and some clinical problems related to lungfluke in China. Annual Report of the Chung Shang Medical College 1958:192-3.
- [Google Scholar]
- The Chinese species of paragonimid trematodes, their phylogenetic relationship and faunal distribution. Acta Parasitologica Sinica. 1964;1:53-68.
- [Google Scholar]
- Studies on a new lung fluke: Paragonimus hueit’ungensis sp. Nov. Chinese Med J. 1977;3:379-94.
- [Google Scholar]
- Illustrated book of Helminthic zoonoses (1st ed). Tokyo: International Medical Foundation of Japan; 1991.
- Phylogenetic reconstruction using secondary structures and sequence motifs of ITS2 rDNA of Paragonimus westermani (Kerbert, 1878) Braun, 1899 (Digenea: Paragonimidae) and related species. BMC Genomics. 2009;10(Suppl 3):S25.
- [Google Scholar]
- Geographical genetic structure within human lung fluke, Paragonimus westermani, detected from DNA sequences. Parasitology. 1997;115:411-7.
- [Google Scholar]
- Paragonimus skrjabini Chen, 1959 (Digenea: Paragonimidae) and related species in eastern Asia: A combined molecular and morphological approach to identification and taxonomy. Syst Parasitol. 2005;60:1-21.
- [Google Scholar]
- A new species of Paragonimus (Trematoda: Troglotrematidae) from a cat infected with metacercariae from mountain crabs Larnaudia larnaudii. J Parasitol. 2007;93:1496-500.
- [Google Scholar]
- Molecular phylogenetic relationship of Paragonimus pseudoheterotremus. Southeast Asian J Trop Med Public Health. 2008;39:217.
- [Google Scholar]
- Paragonimus in Asia: Biology, genetic variation and speciation. In: Kawashima K, ed. Paragonimus Research Report Number 2. Fukuoka, Japan: Kyushu University School of Health Sciences; 1989.
- [Google Scholar]
- Studies on lung fluke, Paragonimus westermani diploid type: In: Northern part of Hyogo Prefecture, Japan. III. Experimental oral infection with metacercariae of rats, with reference to juvenile worms removed from the muscle. In: Jpn J Parasitol. Vol 33. 1984. p. :119-32.
- [Google Scholar]
- Paragonimiasis and tuberculosis, diagnostic confusion: A review of the literature. Trop Dis Bull. 1995;92:R 1-R 26.
- [Google Scholar]
- Experimental infection of laboratory animals with Paragonimus heterotremus metacercariae occurring in Manipur, India. SEA J Trop Med Public Health. 2010;1:47-51.
- [Google Scholar]
- Paragonimus westermani (Szechuan variety) and a new species of lung fluke- Paragonimus szechuanensis, Part II. Studies on clinical aspects of paragonimiasis szechuanensis - a new clinical entity. Chin Med J. 1962;81:419-34.
- [Google Scholar]
- Re-emergence of paragonimiasis in Kyushu, Japan. Southeast Asian J Trop Med Public Health. 1999;30:686-91.
- [Google Scholar]
- Paragonimiasis: A common cause of persistent pleural effusion in Lao PDR. Trans R Soc Trop Med Hyg. 2009;103:1019-23.
- [Google Scholar]
- Pathologic changes of paragonimiasis: A report of five cases. Chin Med J. 1957;75:986-1003.
- [Google Scholar]
- Chronic cerebral paragonimiasis combined with aneurismal subarachnoid haemorrhage. Am J Trop Med Hyg. 2003;69:466-9.
- [Google Scholar]
- Cerebral paragonimiasis. Topics on tropical neurology. Contemporary Neurology Series. 1975;12:109-32.
- [Google Scholar]
- Cerebral paragonimiasis mimicking tuberculoma: First case report in India. Trop Parasitol. 2011;1:39-41.
- [Google Scholar]
- An imaging diagnosis of cerebral paragonimiasis: CT and MR findings and correlation with ELISA antibody test. J Korean Radiol Soc. 1993;29:345-54.
- [Google Scholar]
- Pulmonary paragonimiasis with expectoration of worms: A case report. Southeast Asian J Trop Med Public Health. 1981;12:104-6.
- [Google Scholar]
- The recovery of Paragonimus eggs from stools of paragonimiasis patients by the AMS III centrifugation technique. Jpn J Med Sci Biol. 1953;6:207-12.
- [Google Scholar]
- Pulmonary paragonimiasis: Diagnostic value of pleural fluid analysis. South Med J. 1986;79:241-3.
- [Google Scholar]
- Studies on the immunodiagnosis of paragonimiasis II.Intradermal test with fractionated antigen. J Infect Dis. 1968;118:235-9.
- [Google Scholar]
- Immunological diagnosis as the screening method of paragonimiasis in the endemic area of paragonimiasis. In: Proceedings of the first regional symposium on scientific knowledge of tropical parasites, University of Singapore. Singapore: UNESCO; 1962. p. :194-206.
- [Google Scholar]
- Epidemiology and control of paragonimiasis. In: Sasam M, ed. Parasitic diseases. Tokyo: International Medical Foundation of Japan; 1974. p. :137-49.
- [Google Scholar]
- Contribution of immunoelectrophoretic analysis to the knowledge of worm antigens: Practical effects on their standardization, their purification & diagnosis of helminthiasis by immunoelectrophoresis. Rev Immunol Ther Antimicrob. 1965;29:5-30.
- [Google Scholar]
- Immunological study on paragonimiasis.Agar-gel precipitation reactions in paragonimiasis. Jpn J Trop Med Hyg. 1968;10:28-38.
- [Google Scholar]
- Immunodiffusion test in the diagnosis of paragonimiasis. In: Second international congress of parasitology. Washington D.C.: Colloquium on Immunodiagnosis; 1970. p. :6-12.
- [Google Scholar]
- On the immunoelectrophoresis for helminthological researches. Jpn J Parasitol. 1974;23:335-45.
- [Google Scholar]
- Serodiagnosis of human paragonimiasis caused by Paragonimus heterotremus. Southeast Asian J Trop Med Public Health. 1990;21:103-7.
- [Google Scholar]
- Human immune response of cats to Paragonimus infection. Southeast Asian J Trop Med Public Health. 1981;12:364-7.
- [Google Scholar]
- Evaluation of ELISA for the diagnosis of paragonimiasis westermani. Trans R Soc Trop Med Hyg. 1987;81:3-6.
- [Google Scholar]
- Serodiagnosis of paragonimiasis by enzyme-linked immunosorbent assay and immunoelectrophoresis. Southeast Asian J Trop Med Public Health. 1989;20:243-51.
- [Google Scholar]
- Multidot enzyme-linked immunosorbent assay for serodiagnosis of trematodiasis. Southeast Asian J Trop Med Public Health. 1990;21:471-4.
- [Google Scholar]
- Enzyme-linked immunosorbent assay using cysteine proteinase antigens for immunodiagnosis of human paragonimiasis. AmJ Trop Med Hyg. 1996;55:434-7.
- [Google Scholar]
- Studies on immunodiagnosis of human paragonimiasis and specific antigen of Paragonimus heterotremus. Int J Parasitol. 1991;21:395-401.
- [Google Scholar]
- Characterization and localization of Paragonimus westermani antigen stimulating antibody formation in both the infected cat and rat. J Parasitol. 1987;73:363-7.
- [Google Scholar]
- Partially purified antigens of Paragonimus heterotremus for serodiagnosis of human paragonimiasis. Southeast Asian J Trop Med Public Health. 1994;25:176-80.
- [Google Scholar]
- Human lung fluke Paragonimus heterotremus: Differential diagnosis between Paragonimus heterotremus and Paragonimus westermanif infections by EITB. Trop Med Int Health. 1998;3:52-6.
- [Google Scholar]
- Diagnosis of active Paragonimus westermani infections with monoclonal antibody-based antigen detection assay. Am J Trop Med Hyg. 1993;49:329-34.
- [Google Scholar]
- Parasite specific IgE and IgG levels in the serum and pleural effusion of paragonimiasis westermani patients. Am J Trop Med Hyg. 1992;47:104-7.
- [Google Scholar]
- Clinical features of paragonimiasis cases recently found in Japan: parasite-specific immunoglobulin M and G antibody classes. Clin Infect Dis. 2001;32:171-5.
- [Google Scholar]
- Development of enzyme – linked immunosorbent assay for serodiagnosis of human paragonimiasis. Indian J Med Res. 2005;121:739-46.
- [Google Scholar]
- Development of rapid diagnostic kit (Dot Immunogold Filtration Assay) for detection of antibodies against Paragonimus westermani. Chinese J Zoon. 2005;21:988-90.
- [Google Scholar]
- Radiological findings in pulmonary paragonimiasis heterotremus. Southeast Asian J Trop Med Public Health. 1984;15:122-8.
- [Google Scholar]
- Pulmonary paragonimiasis: An evaluation of roentgen findings in 38 sputum positive patients in an endemic area in Thailand. Am J Roentgenol. 1959;81:236-44.
- [Google Scholar]
- Pulmonary patragonimiasis: Clinical and experimental studies. Radiographics. 1993;13:575-86.
- [Google Scholar]
- Cerebral paragonimiasis in early stage: CT and MR features. AJR Am J Roengenol. 1994;162:141-5.
- [Google Scholar]
- Chemotherapeutic effect of niclofan and praziquantel in the treatment of pulmonary paragonimiasis. Korea Univ Med J. 1980;17:113-8.
- [Google Scholar]
- Recent progress in studies of Paragonimus and paragonimiasis control in China. Chin Med J. 1981;94:483-94.
- [Google Scholar]
- Assessment of efficacy, safety, and tolerability of praziquantel and triclabendazole in the treatment of paragonimiasis. Southeast Asian J Trop Med Public Health. 2007;38(Suppl 1):97-105.
- [Google Scholar]
- Comparison of two single day regimens of triclabendazole for the treatment of human pulmonary paragonimiasis. Trans Roy Soc Trop Med Hygiene. 2003;9:451-4.
- [Google Scholar]
- Paragonimiasis: An emerging public health problem in India. Indian J Med Res. 2005;121:716-8.
- [Google Scholar]






