Translate this page into:
Absolute lymphocyte count as a surrogate marker for CD4 counts after six months of HAART initiation in a resource-limited setting in India
Reprint requests: Dr S. Srirangaraj, Associate Professor, Department of Microbiology, Mahathma Gandhi Medical College & Research Institute, Puducherry 607 402, India e-mail: rangaraj1980@indiatimes.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Owing to the ever-expanding access to HAART (highly active anti-retroviral therapy) in resource-limited settings, there is a need to evaluate alternate markers like absolute lymphocyte count (ALC) as a surrogate for CD4 counts. This study was done to assess the usefulness of ALC as a surrogate marker for CD4 counts in monitoring HIV-infected patients after HAART initiation.
Methods:
In this study, 108 HIV-positive adult patients of both sexes fulfilling the inclusion criteria were included. CD4 and ALC were recorded at baseline. After initiation on HAART, these patients were followed up at three month intervals.
Results:
ALC and CD4 counts were positively correlated (Spearman correlation coefficient= 0.553). After six months of HAART, the sensitivity of an ALC increase as a marker for CD4 count increase at six months was 82 per cent, specificity was 100 per cent, PPV was 100 per cent and NPV was 31 per cent. Area under the corresponding ROC curve for CD4 increase of >100 cells/μl was 0. 825 ± 0.053.
Interpretation & conclusions:
ALC may be a useful surrogate marker in predicting an increase in CD4 counts as a response to HAART, but of questionable value in predicting a decrease in CD4 counts.
Keywords
Absolute lymphocyte count
CD4 counts
HAART
surrogate marker
In India, with the increasing availability of generic anti-retroviral drugs through the National AIDS Control Organization (NACO), there is an increasing need for effective monitoring of therapy12. Current recommendations in western countries for initiation and monitoring of HAART (highly active anti-retroviral therapy) in patients infected with HIV are based on CD4+ T-cell counts and plasma HIV RNA levels (viral load)3. However, these standard methods require highly trained personnel and heavy initial investment in laboratory instrumentation2. So, there is a need to evaluate alternate markers like absolute lymphocyte count (ALC) as a surrogate for CD4 count, as the cost of CD4 count estimation is 45 times higher than ALC4.
Numerous studies from India, Africa and the western world have established the role of total lymphocyte counts (TLC) as surrogate for CD4 counts for starting opportunistic infection prophylaxis5–8, in determining when to initiate anti-retroviral therapy910, and in monitoring patients on ART211. According to the WHO guidelines, the TLC is no longer recommended to guide treatment decisions in adults and adolescents12. However, no mention has been made of ALC in these recommendations. There are also a few studies suggesting that the ALC might be useful in identifying patients who would benefit from initiating prophylaxis for AIDS-related opportunistic infections or in deciding when to initiate HAART913. A study from Africa reported the utility of ALC as a surrogate for CD4 after HAART initiation14, but there have been no studies published from India.
Hence, the present study was conducted in a resource-limited setting in Karnataka, a State in south India, to assess the usefulness of ALC as a surrogate for CD4 counts in HIV positive patients after HAART initiation.
Material & Methods
Study design and setting: This study was conducted at the ART Centre at Mysore Medical College and Research Institute, Mysore, which caters to Mysore and surrounding districts like Kodagu, Hassan and Chamrajanagar in Karnataka. A total of 108 randomly selected newly registered ART-naïve HIV positive patients, attending the ART Centre, from August 2006 to 2007, were included in the study.
Selection and description of participants: After taking an informed consent (for HIV testing), these individuals, voluntarily attending the ICTC (Integrated Counselling and Testing Centre) at the Department of Microbiology, underwent pre-test counselling, followed by HIV testing as per the strategy III of the NACO guidelines15. After post-test counselling, those found HIV positive, were referred to the ART Centre.
Inclusion & exclusion criteria: HIV infected adults, above 18 yr of age from both sexes, who satisfied the following criteria were included in the study: (i) WHO stage IV disease irrespective of CD4 cell counts, or WHO stage III disease with CD4 cell counts <350 cells/μl or WHO stage I or II disease with CD4 cell counts <200 cells/μl16, (ii) not on prior anti-retroviral therapy, (iii) having haemoglobin (Hb) value of >10 g/dl (for Zidovudine-based regimens). If Hb was <10 g/dl, patient was put on d4T (Stavudine)-based regimens, and (iv) Alanine transmitase (ALT) level no more than five times the upper limit of normal (normal levels 0-45 IU/l at 37°C), a total bilirubin concentration that did not exceed 2.5 mg/dl, a serum creatinine concentration of no more than 2 mg/dl.
Patients who had symptoms of pancreatitis or peripheral neuropathy were excluded.
All patients were initiated on various HAART regimens by strictly following the NACO guidelines17. The common first-line regimens used were zidovudine (AZT) plus lamivudine (3TC) plus nevirapine (NVP); followed by stavudine (d4T) plus lamivudine (3TC) plus nevirapine (NVP); zidovudine plus 3TC plus efavirenz (EFV); and d4T plus 3TC plus EFV.
CD4 enumeration: Using standard precautions, 4 ml of venous blood was collected between 0900 to 1200 h using a 2 ml K3-EDTA Vacutainers (Becton, Dickinson and Company, San Jose, United States of America). The CD4/CD3 enumeration was done using the single–platform BD FACS Calibur™ machine (Becton, Dickinson and Company, San Jose, United States of America), by following the manufacturer's instructions. Internal quality control was performed with process controls using the manufacturer's recommendations. External quality control was performed through an external quality assurance programme with NARI (National AIDS Research Institute), Pune, India.
For each patient, the clinic visit prior to initiating a new HAART regimen served as the baseline, during which the CD4 counts and ALC were recorded. After initiation on HAART, these patients were followed up at regular intervals of every 3 months until the end of the study period. Data evaluated in the present study were from the 6th month follow up visit, when both CD4 counts and ALC were done.
The study protocol was approved by the ethics Committee of the institute.
Statistical analysis: Two specific strategies were evaluated for using ALC change as a diagnostic marker for CD4 change. In the first part of the analysis, sensitivity, specificity, positive and negative predictive values (PPV and NPV) were calculated. Correlation between ALC and CD4 counts was calculated by Spearman's correlation coefficient and also by the coefficient of determination. In the second part of the analysis, the diagnostic capability of absolute change in ALC was evaluated as a marker of 6-month CD4 change. This was done by calculating the receiver operator characteristic (ROC) curves18.
To determine the best separator (a continuous variable), ROC curves were plotted for different CD4 count changes of 50, 100, 150, 200, 250, 300, 350 and 400 cells/μl (as separator variables). The areas under the corresponding ROC curves were recorded and the separator (i.e., the CD4 change of ‘x’ cells) at which the area under the ROC curve was highest was selected as the CD4 count cut-off. Further, to determine an ALC cut-off for those using ALC alone as a marker, in absence of CD4 counts, sensitivity, specificity, PPV, NPV, and likelihood ratio (LR) were calculated for change in ALC of 200, 400, 600, 800 and 1000 cells/μl as a marker for 6-month change in the previously identified CD4 cut-off and the results were tabulated.
All statistical analyses were performed using SPSS software (version 16.0, SPSS, Chicago, USA).
Results
Of the 108 patients, 71 (65.74%) were males and 37 (34.26%) were females. The mean CD4 count and mean ALC at baseline were 130 ± 77 and 1348 ± 761 cells/μl, respectively. Only 100 patients were initiated on HAART. As the study period was restricted to one year, only four patients completed follow up of one year. Due to this small sample size, the analysis was restricted to follow up at 6 months as 66 patients completed the 6-month follow up.
Fig. 1 shows box plots of change in ALC corresponding to categories of change in CD4 count at the 6th month visit. A positive relationship was evident. For example, for occasions where CD4 count change from baseline was +100 to +150 cells/μl, the median of the corresponding change in ALC was +249 cells/μl (-99 to 1254 cells/μl, inter quartile range), when CD4 count improved by more than 150; the median concomitant change in ALC was +889 cells/μl (390.75 to 1175 cells/μl, interquartile range).
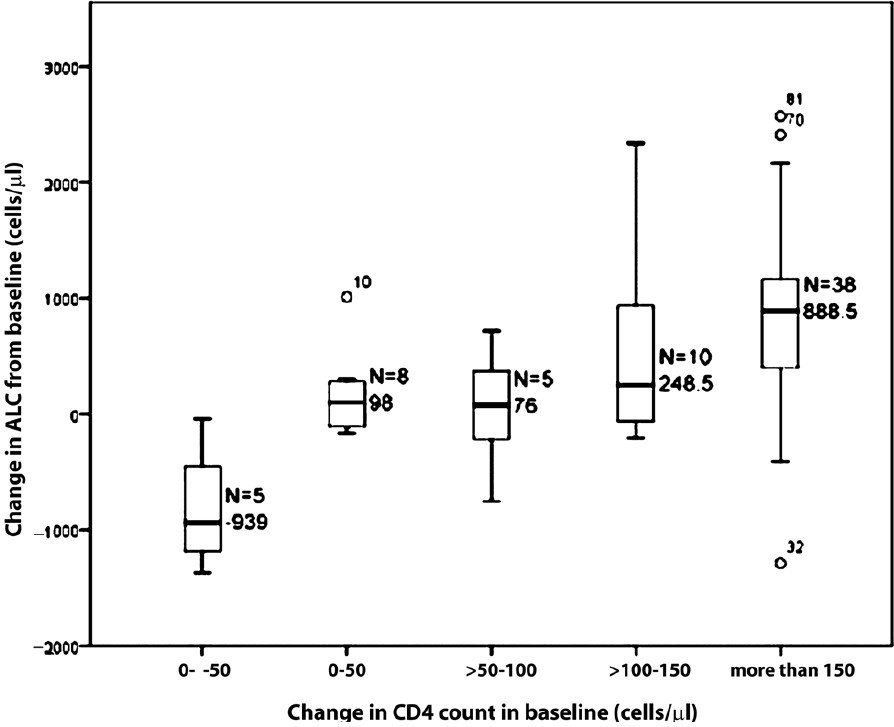
- Box plot of change in ALC (cells/μl) from baseline stratified by change in CD4 count (cells/μl) from baseline at 6th month ALC. Note- The numbers are very small on account of the small sample size.
Similarly, decreases in CD4 were also associated with decreases in ALC. For example, when CD4 count change from baseline was 0 to -50 cells/μl, the median of the corresponding change in ALC was -939 cells/μl (-1281 to -247 cells/μl, interquartile range).
Sixty six paired ALC and CD4 counts had a Spearman correlation coefficient of 0.553 (corrected for ties) (95% confidence interval: 0.3525 to 0.7048, two-tailed P<0.0001). Also, the coefficient of determination, an index with a clearer operational interpretation than the coefficient of correlation was equal to 0.506.
Fig. 2 shows scatter plot of the changes in CD4 count versus corresponding changes in ALC after 6-months of antiretroviral therapy. Each point on the plot represents a CD4 count and ALC pair. A positive association was seen between change in ALC and change in CD4 count at 6-month interval of HAART.
During the first 6 months of treatment, sensitivity was 0.82 and the specificity was 1.00. Prevalence (depending on the proportion of occasions on which CD4 increased) for our study was 0.92. PPV was found to be 1.00 and NPV was 0.31.
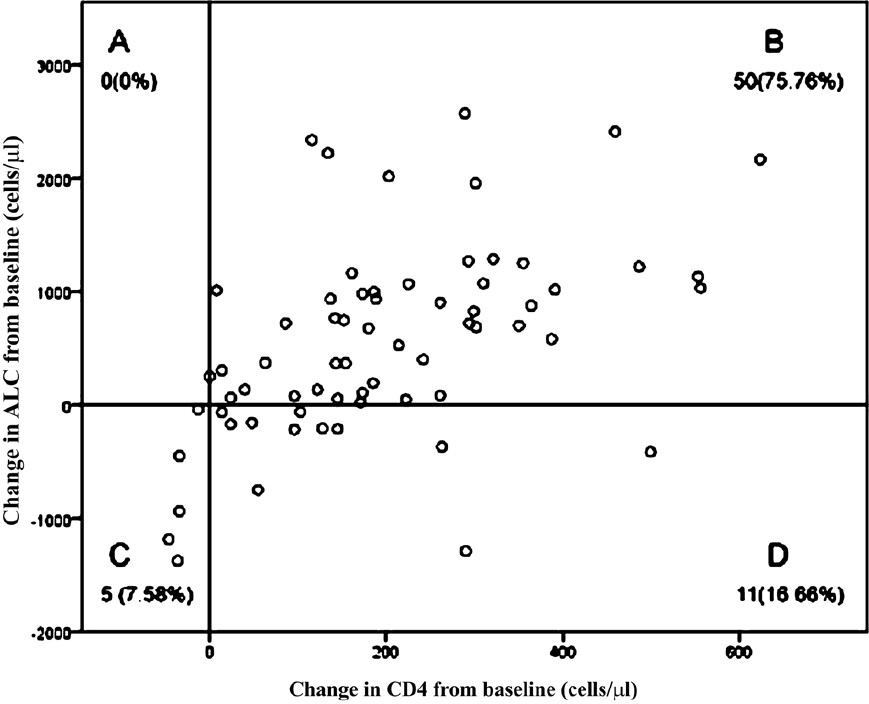
- Scatter plot of change from baseline in ALC vs. corresponding change from baseline in CD4 at 6-month intervals during the first 6 months of antiretroviral therapy. Each plot divided into quadrants (A-D). The number (%) in each quadrant is listed on each plot. Quadrant A: CD4 count decreased with ALC increase. Quadrant B: CD4 count increased with ALC increase. Quadrant C: CD4 count decreased with ALC decrease. Quadrant D: CD4 count increased with ALC decrease.
ROC curves were calculated for changes in CD4 count of 50, 100, 150, 200, 250, 300, 350 and 400 cells/μl. The 6-month CD4 change of >100 cells/μl had the highest area under the ROC curve, thus, becoming the best separator variable (Table I). Hence, CD4 change of >100 cells/μl was taken as the cut-off. The area under the corresponding receiver operator curve for change in ALC as a marker for 6-month change in CD4 count of >100 cells/μl was 0.825 (95% C.I. = 0.721-0.928), indicating strong diagnostic capability (Fig. 3).

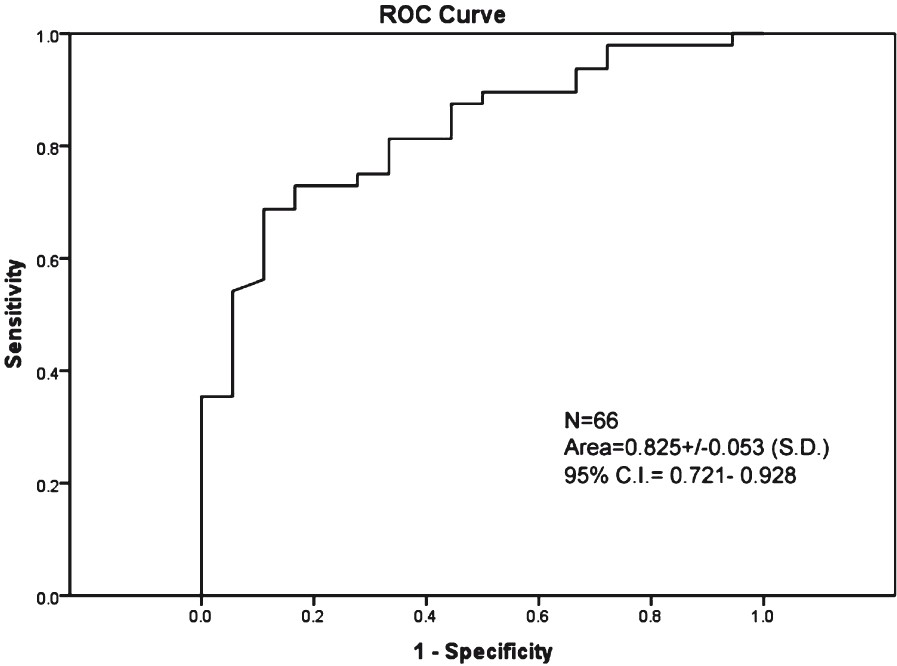
- ROC curves based on using absolute 6-month change in ALC as a marker for 6-month CD4 change of >100 cells/μl.
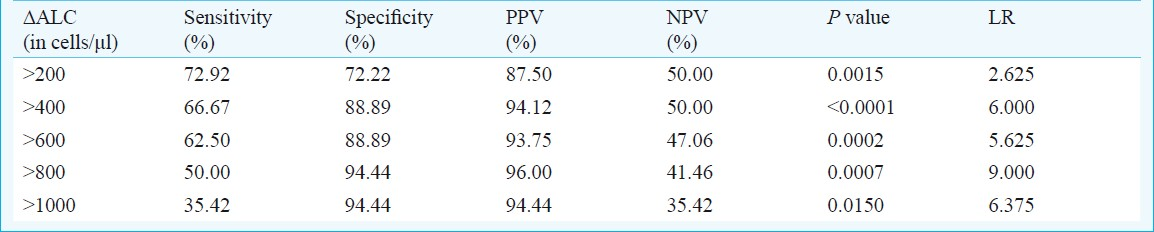
The sensitivity, specificity, PPV, NPV and likelihood ratio (LR) for varying thresholds of ALC change (ΔALC) as a marker for the cut-off (6-month) CD4 change of >100 cells/μl is given in Table II. Sensitivity was maximum at ΔALC >200 cells/μl (two-sided P=0.0015). Positive predictive value was highest at ΔALC >800 cells/μl (96%).
Discussion
In this study, the usefulness of absolute lymphocyte count as a surrogate for CD4 counts after HAART initiation was studied. Though earlier studies exist on the usefulness of TLC as a surrogate for CD4 after HAART initiation219, TLC has not found universal acceptance as a surrogate for CD4 counts. Akinola et al20 showed that TLC was not a reliable predictor of CD4 cell count in HIV-infected individuals. The latest WHO guidelines12 no longer recommends TLC as a guide to make treatment decisions in adults and adolescents.
ALC refers to the CD45+ T-cell count and has already been shown to be a useful marker for CD4 in initiation of HAART913. There have been a few studies on ALC as surrogate for CD4 counts after HAART initiation showing equivocal evidence as regards its usefulness. Akanmu et al14 have concluded that ALC correlates weakly with CD4 counts in patients undergoing antiretroviral therapy and it may not serve as a perfect surrogate for CD4 as a monitor of immunological response to therapy. Jibrin et al21 did not recommend the use of ALC as a surrogate for CD4 counts, due to the weak correlation between the two. Both these studies had the drawback of using small sample sizes to arrive at their conclusions.
In our study, a positive ALC change is shown to be a sensitive and specific marker of positive CD4 change. For settings where the probability of positive CD4 change is high, an ALC increase is almost perfectly predictive of a CD4 increase (PPV=100%), however, an ALC decrease is only modestly predictive of a true CD4 decrease (NPV=31%). The implication is that in a clinical setting, whenever, ALC decreases, other tests like CD4 counts may have to be done to confirm the findings. Though the number of tests will go up in case the ALC decreases in a patient, we believe if cheaper methods of ALC estimation can be validated, the overall cost will still be less as only a small portion of patients with ALC decrease will require CD4 estimation (compared with the present situation, where CD4 counts are performed in all patients).
The area under the ROC showed the diagnostic utility of ALC as a meaningful surrogate for changes in CD4 count in response to HAART. As per the WHO guidelines on ART (2010)12, there is no mention of a specific increase in CD4 counts as relevant, though the 2002 guidelines22 mentioned a CD4 increase of 50 cells/μl as clinically relevant.
We calculated ROC curves for various CD4 count increases and selected the separator variable at which the area under the corresponding ROC curve was the highest, as the CD4 cut-off. We tried to determine a cut-off value for those clinicians, using ALC alone as a marker in absence of CD4 counts, by varying thresholds of change in ALC as a marker for 6-month change in CD4 of >100 cells/μl. The sensitivity, specificity, PPV, NPV and likelihood ratio aggregated maximally at a ΔALC of >400 cells/μl. However, this cut-off value should be interpreted with caution as measurement error between different instruments may vary from location to location and the decision regarding the desirability of having a false-positive/ negative result will have to be considered bearing in mind the cost considerations.
Another important aspect to be considered is the variability of CD4 counts on account of factors like race, sex, diurnal variation, intra- and inter-instrument variation. All the patients in our study were native of Mysore and surrounding districts, hence belonged to the same race. In an earlier study, we had established the normal CD4 range in males and females in our region, to account for the variation in CD4 counts in males and females23. All the samples were collected between 0900 to 1200 h to offset the diurnal variations in CD4 counts. For intra-instrument variations in CD4 counts, we had a quality control programme. However, more studies need to be done to assess the impact of inter-instrument variation on ALC assessment and in turn its role in CD4 surrogacy.
The small sample size of the study was the major limitation. Hence, more studies with larger sample sizes need to be done to corroborate or refute these findings. Other factors affecting the interpretation included the possibility of leucocytosis owing to intercurrent infections. Cost-effectiveness analysis could not be done in our study as both TLC and CD4 estimations were done by the same instrument. Another limitation of this study was the short duration of the study period, which precluded the possibility of categorizing our patients as short term progressors, rapid progressors or long-term non-progressors, as such a categorization would require a longer duration of study.
There are no data regarding the role of ALC in characterization of long term non progressor (LTNP), rapid progressor or slow progressor in HIV infection, but there is one study from India on the use of CD4+ T-lymphocyte percentage and a plasma viral load of <6000 copies per ml as criteria for defining LTNPs24. More studies are required to establish the role of ALC, if any, in categorization of progressors. Also, more region-specific validation of CD4 and ALC changes on HAART are needed to remove disparities of sensitivities and specificities of ALC as a proxy for CD4 count.
In summary, ALC may be a useful surrogate marker in predicting an increase in CD4 counts as a response to HAART, but of questionable value in predicting a decrease in CD4 counts.
References
- CD4+ T cell count as a tool to monitor HIV progression & anti-retroviral therapy. Indian J Med Res. 2005;121:539-49.
- [Google Scholar]
- Changes in total lymphocyte count as a surrogate for changes in CD4 count following initiation of HAART: implications for monitoring in resource-limited settings. J Acquir Immune Defic Syndr. 2004;36:567-75.
- [Google Scholar]
- Rates of disease progression by baseline CD4 cell count and viral load after initiating triple drug therapy. JAMA. 2001;286:2568-77.
- [Google Scholar]
- Absolute lymphocyte count is a useful marker of HIV-1 disease progression in HIV-1 infected individuals in Pune, India. Int Conf on AIDS 2004 July 11-16
- [Google Scholar]
- Total lymphocyte count as a possible surrogate of CD4 cell count to prioritize eligibility for antiretroviral therapy among HIV-infected individuals in resource-limited settings. Antivir Ther. 2003;8:379-84.
- [Google Scholar]
- Total lymphocyte count (TLC) is a useful tool for the timing of opportunistic infection prophylaxis in India and other resource-constrained countries. J Acquir Immune Defic Syndr. 2002;31:378-83.
- [Google Scholar]
- Total lymphocyte count as a tool for timing opportunistic infection prophylaxis in resource-limited settings: a study from India. J Infect Dev Countries. 2010;4:645-9.
- [Google Scholar]
- Total lymphocyte count as a predictor of absolute CD4+ count and CD4+ percentage in HIV-infected persons. JAMA. 1993;269:622-6.
- [Google Scholar]
- Absolute or total lymphocyte count as a marker for the CD4 T lymphocyte criterion for initiating antiretroviral therapy. AIDS. 2003;17:917-9.
- [Google Scholar]
- Predicting CD4 count using total lymphocyte count: a sustainable tool for clinical decisions during HAART use. Am J Trop Med Hyg. 2005;73:58-62.
- [Google Scholar]
- Use of total lymphocyte count for monitoring response to antiretroviral therapy. Clin Infect Dis. 2004;38:257-62.
- [Google Scholar]
- World Health Organization. In: Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach, 2010 revision. Austria: WHO Press; 2010. p. :1-156.
- [Google Scholar]
- Correlation amount total lymphocyte count, absolute CD4+ count and CD4+ percentage in a group of HIV-1-infected South African patients. J Acquir Immune Defic Syndr. 1998;19:238-44.
- [Google Scholar]
- Absolute lymphocyte count as surrogate for CD4+ cell count in monitoring response to antiretroviral therapy. Niger Postgrad Med J. 2001;8:105-11.
- [Google Scholar]
- National AIDS Control Organization. In: HIV testing manual: Laboratory diagnosis, biosafety and quality control. New Delhi: National AIDS Control Organization; 2001.
- [Google Scholar]
- World Health Organization. In: Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. France: WHO Press; 2006.
- [Google Scholar]
- National AIDS Control Organization. In: HIV sentinel surveillance and HIV estimation- A technical brief. New Delhi: NACO; 2008.
- [Google Scholar]
- Introductory biostatistics. Hoboken, New Jersey: John Wiley and Sons, Inc; 2003. p. :116-7.
- Usefulness of total lymphocyte count in monitoring highly active antiretroviral therapy in resource-limited settings. AIDS. 2003;17:541-5.
- [Google Scholar]
- The search for a predictor of CD4 cell count continues: total lymphocyte count is not a substitute for CD4 cell count in the management of HIV-infected individuals in a resource-limited setting. Clin Infect Dis. 2004;39:579-81.
- [Google Scholar]
- Should absolute lymphocyte count be used as a surrogate marker for CD4+ count in patients with HIV/AIDS? Afr J Med Med Sci. 2006;35:9-13.
- [Google Scholar]
- World Health Organization. In: Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach. Geneva: WHO; 2003.
- [Google Scholar]
- Normal CD4 and CD3 lymphocyte counts in healthy south Indian adults. Indian J Med Microbiol. 2010;28:183-4.
- [Google Scholar]
- Laboratory characteristics of HIV-1 clade C-infected long-term non-progressors at a tertiary human immunodeficiency virus care centre in South India. J Med Microbiol. 2008;57:913-5. 1
- [Google Scholar]






