Translate this page into:
Establishment & characterization of lymphoblastoid cell lines from patients with multiple primary neoplasms in the upper aero-digestive tract & healthy individuals
Reprint requests: Dr Rita Mulherkar, KS-352, Khanolkar Shodhika, ACTREC, Tata Memorial Centre, Kharghar, Navi Mumbai 410 210, India e-mail: rmulherkar@actrec.gov.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
A major drawback for genetic studies as well as long-term genotype-phenotype correlation studies in cancer is lack of representative human cell lines providing a continuous source of basic biomolecules and a system to carry out various experimental investigations. This can be overcome to some extent by establishing lymphoblastoid cell lines (LCLs) by infecting peripheral blood lymphocytes with Epstein Barr virus (EBV) which is known to immortalize human resting B cells in vitro giving rise to actively proliferating B-lymphoblastoid cell lines. The present study involves preparation and characterization of LCLs generated from patients with multiple primary neoplasms (MPN) of upper aero-digestive tract (UADT).
Methods:
Thirty seven LCLs were established from UADT MPN patients and healthy age, sex and habit matched controls using EBV crude stock. Characterization was done with respect to expression of CD-19 (Pan B-cell marker), CD3 (T cell specific marker), CD56 (NK-cell specific marker), cell morphology, ploidy analysis, genotype and gene expression comparison with the parent lymphocytes.
Results:
LCLs showed rosette morphology with doubling time of approximately 24 h. Ploidy analysis showed diploid DNA content which was maintained for at least 30 population doublings. When compared with parent lymphocytes there appeared no change at genetic and gene expression level.
Interpretation & conclusions:
Our results show that lymphoblastoid cell lines are a good surrogate of isolated lymphocytes bearing their close resemblance at genetic and phenotypic level to parent lymphocytes and are a valuable resource for understanding genotype-phenotype interactions.
Keywords
Epstein Barr virus
lymphoblastoid cell lines
multiple primary neoplasia
ploidy analysis
population doubling
Squamous cell carcinoma of the upper aero-digestive tract (UADT), comprising head, neck, oesophagus, trachea and lungs, are common cancers worldwide and one of the most common cancers found in Indian men1. Further, a 3-7 per cent annual risk of development of a second primary neoplasm among the survivors of early stage UADT cancer poses an additional threat in terms of morbidity and mortality2. Cumulative evidence from the case-control studies analyzing polymorphisms in xenobiotic metabolism, DNA repair and other gatekeeper mechanisms suggest aberrant gene-environment interactions to be an important aetiological factor in the genesis of multiple primary neoplasm (MPN)34. Thus it is becoming increasingly important to validate the findings of huge number of genotyping studies in at least a subset of the patients by phenotypic assays. Short term studies to assay chromosomal aberrations, mutagen sensitivity assays and DNA repair kinetics have been carried out using lymphocytes or short term lymphocyte cultures derived from study population5–7. However, limited availability of biological material and lack of reproducibility has been the major limitation of such studies.
Immortalization of human B lymphocytes by Epstein Barr virus (EBV) in vitro is used routinely to establish lymphoblastoid cell lines (LCLs). Infection by EBV transforms resting B cells from human peripheral blood into actively proliferating LCLs8. Unlike SV40 or human papilloma virus (HPV), EBV allows cell immortalization with minimal genetic and phenotypic aberrations, with ease of establishment and maintenance making LCLs ideal material for genotypic and phenotypic studies8. These provide an unlimited source of biomolecules like DNA, RNA or proteins and are a promising in vitro model system for genetic screening studies, genotype-phenotype correlation studies, a variety of molecular and functional assays along with immunology and cellular biology studies9–12. Utility of LCLs is also been very well documented in various population based studies especially investigating in vitro carcinogen sensitivity and DNA damage/repair13–15. Here we report generation of 37 LCLs from patients with UADT MPN and healthy individuals and characterization of a few randomly selected cell lines.
Material & Methods
This study was approved by the Hospital Ethics Committee, Tata Memorial Centre, Mumbai and conducted during 2005-2009 in the Mulherkar laboratory of Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Navi Mumbai, Maharashtra, India. Samples from healthy, age, sex and habit matched, control individuals were obtained from staff and students of ACTREC, Navi Mumbai.
Collection of samples: After obtaining IRB approval and patient informed consent, 3 ml whole blood was collected in an EDTA vacutainer from patients with MPN and cancer free healthy individuals by venipuncture. Patients with MPN were accrued from the Cancer Genetics Clinic in Tata Memorial Hospital and samples from healthy control individuals were obtained from Advanced Center for Treatment Research and Education in Cancer, Mumbai, India. The study was approved by the Hospital Ethics Committee, Tata Memorial Hospital, Mumbai. UADT MPN patients were recruited on the basis of criteria given by Hong et al16 as described earlier17.
Generation of viable EBV stock: EBV-transformed B95-8 marmoset cell line was procured from National Centre for Cell Sciences, India and the EBV stock prepared. Briefly, 0.5×106 cells/ml were seeded in RPMI-1640, 15 per cent foetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 200 mM glutamine (Sigma-Aldrich Co., USA) and 1X PenStrep (Sigma-Aldrich Co., USA). After 7 days confluent cultures of B95-8 appearing straw yellow in colour were lysed by freeze thawing at -80°C and 37°C and filtered through 0.22 μM filter (Millipore, Bedford, MA) to obtain EBV crude stock. The filtrate was aliquoted and stored at 4°C for short term or -80°C for long-term storage.
Lymphoblastoid cell line preparation: For separation of peripheral blood lymphocytes (PBLs) approximately 3 ml of blood was separated on a Ficoll-Hypaque gradient (Sigma-Aldrich Co., USA). PBLs were seeded in a sterile 24 well plate at a density of 1.5-2×106 cells/ml in Dulbecco's minimal essential medium (DMEM) containing 15 per cent FBS 200 m M glutamine and IX Pen Strep. EBV crude stock at 1:1 ratio was added and placed in an incubator maintained at 37°C with 5 per cent CO2. After 24 h medium containing viral supernatant was aspirated without disturbing the cells and fresh complete DMEM was added. After 3-4 wk of incubation rosette morphology of cells ascertained the transformed phenotype of PBLs. Cells were mixed thoroughly to break clumps before splitting to ensure multiclonal population. All the used plasticware (Nunc, Denmark) was treated as biohazard, discarded in 1 per cent sodium hypochlorite after use and autoclaved wherever required.
Characterization of LCL: Characterization of LCLs was done using standard techniques as described below. For all assays freshly grown cells with >90 per cent viability were harvested and washed in 1X PBS at 1500 rpm and used for the genotypic and phenotypic characterization.
Immunophenotyping: 1×106 cells from LCLs were incubated with primary mouse anti-CD3 (T cell marker), anti-CD19 (pan-B cell marker) and anti-CD56 (NK cell marker) antibodies (BD Pharmingen, BD Biosciences, USA) for 45 min on ice. The cells were washed thrice with FACS buffer (PBS containing 1% FBS and 0.02% Na-azide) and incubated for 45 min on ice with secondary goat anti-mouse FITC antibody (antibodies used in this experiment were kind gift from Dr Shubhada Chiplunkar and Dr Naren Joshi, Immunology department, ACTREC). Cells were passed through BD™ 1ml 26G ½ syringe (BD, Singapore) to break any cell aggregates or clumps and analyzed on Flow Cytometer (FACS Calibur, BD Biosciences, USA) at 488 nm excitation. A minimum of 10,000 events were analyzed for each sample. Cellular debris was removed by gating on Forward vs. Side Scatter. Statistical analysis was done using CELLQUEST software (BD Biosciences, USA).
Ploidy analysis of LCLs: LCLs (1×106) and control PBLs (1×106) from healthy volunteer were fixed in 70 per cent ethanol at 4°C for 1 h. The cells were washed twice in PBS followed by incubation with propidium iodide (Sigma-Aldrich Co., USA) and RNaseA (Sigma-Aldrich Co., USA) for 30 min at 37°C. Fluorescence was acquired on Flow Cytometer at 488 nm excitation. Cells were passed through BD™ 1ml 26G ½ syringe before acquisition to break any cell aggregates or clumps and a minimum of 10,000 events were analyzed for each sample. Data were analyzed using ModFit LT V 2.0 software (BD Biosciences, USA). DNA ploidy is defined as diploid DNA represented as single G0/G1 peak on a histogram corresponding to the same DNA content represented as single G0/G1 at the same position in the histogram of the control. Ploidy was measured by calculating DNA index (DI) which is the ratio between the channel number of G0/G1 peak on histogram of the cell line to the channel number of G0/G1 peak of control PBLs.
Expression of ATM gene: RNA was isolated from cell lines and lymphocytes isolated from the same subjects by TRIzol (Invitrogen, Carlsbad, CA) extraction method. β actin PCR18 was performed on isolated RNA to ensure any DNA contamination and samples were treated with DNase using DNA-free kit (Ambion, Austin, TX) wherever required. cDNA was synthesized using 3 μg of total RNA using Superscript First-Strand synthesis system by RT-PCR (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. Expression of ATM gene was measured semi-quantitatively by RT-PCR using gene specific primers (Sigma-Aldrich Co., India; Forward 5’-TGTCATTACGTAGCTTCTCC-3’; Reverse 5’-GCTGAGTAATACGC AAATCC-3) and β actin was used as loading control. PCR products were run on 2 per cent agarose gel and stained with ethidium bromide.
Cell population doubling: 5×104 cells from LCLs were seeded in a 24 well plate with 1.5 ml of complete medium. Viable cell count was taken using Trypan blue dye exclusion method19 at different time points including 0, 12, 24, 36, 48, 72 and 96 h. For each time point four readings were taken.
Results
Establishment of LCLs and morphological analysis: Blood samples from MPN patients (n=24) and cancer free control individuals (n=13) were obtained and subjected to LCL preparation. The demographics of the patients and control individuals are shown in Table I. Considerable cell death of the PBLs was observed after 24 h post EBV infection; however, virus infection promoted B cells to re-populate the culture. The time taken for each LCL preparation varied. On an average culturing the cells 3-4 wk post infection was sufficient to produce >1 million cells.

The average population doubling (PD) time of a few representative LCLs was found to be 24 h, ranging from 12 to 36 h (Fig. 1). The LCLs grew as clusters exhibiting typical rosette morphology in suspension cultures, but single cells were also observed having big nucleus and numerous vacuoles (Fig. 2a, 2b). EBV is known to specifically infect B cells allowing their growth in culture hence the transformed phenotype is expected to have homogeneous cell population, however, flow cytometric analysis revealed the presence of dual population (Fig. 2c). On the basis of morphology and granularity it was revealed that lower (R1) population represents mono cell suspension of interest and the other (R2) population probably represents fraction of cells that have spontaneously differentiated into smaller lymphoid cells with shrunken nucleus that ultimately undergo apoptosis during conventional cell culture thus representing diverse size and granularity20.
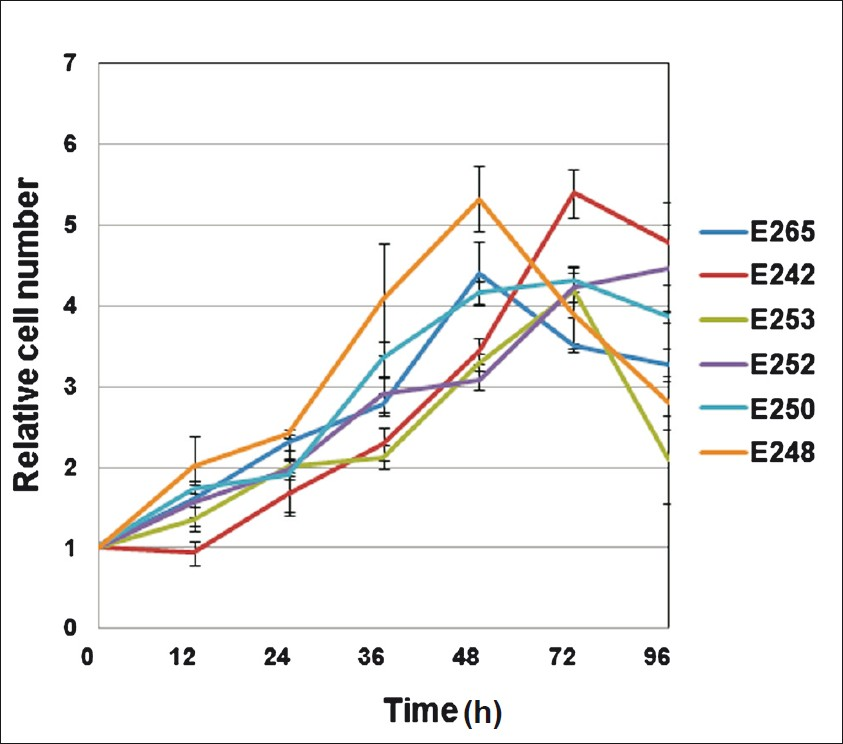
- Graph showing cell population doubling time for 6 representative cell lines at 6 time points till 96 h. The doubling time ranged from 12 to 36 h with an average of approximately 24 h. Values are ± mean SEM (n=4).
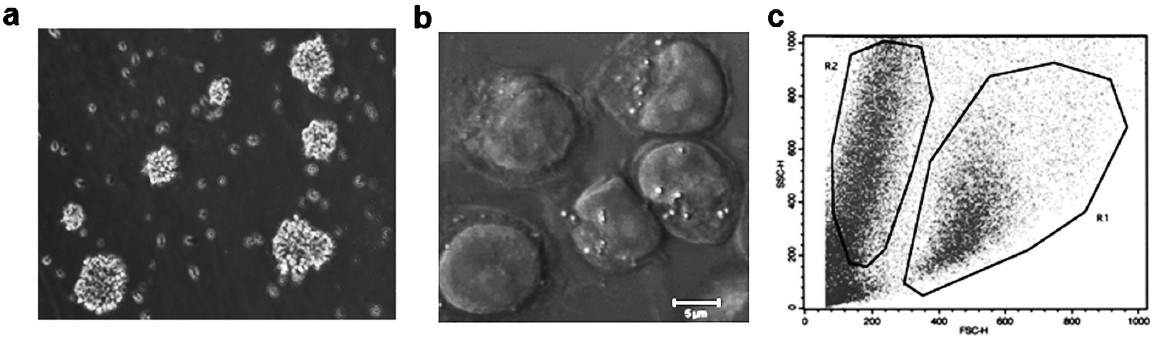
- Morphological analysis of established lymphoblastoid cell lines (a) Light microscopy image of LCL showing typical rosette morphology. Cells grow in clumps while single cells are also seen. (b) Confocal microscopy image of cells in EBV transformed cell lines showing big nucleus and numerous vacuoles. (c) Flow cytometry cluster plot showing two distinct populations in LCL where lower R1 population represents mono cell suspension and R2 population represents cell aggregates.
Cell surface marker: Immunophenotyping was done using PBL as positive control for B (CD19), T (CD3) and NK (CD56) cells. Data confirmed that cells from representative randomly selected LCLs showed expression of typical B cell surface marker (CD19) while markers for T cell (CD3) and NK cells (CD56) were absent (Fig. 3), thus ascertaining the purity of growing cultures.
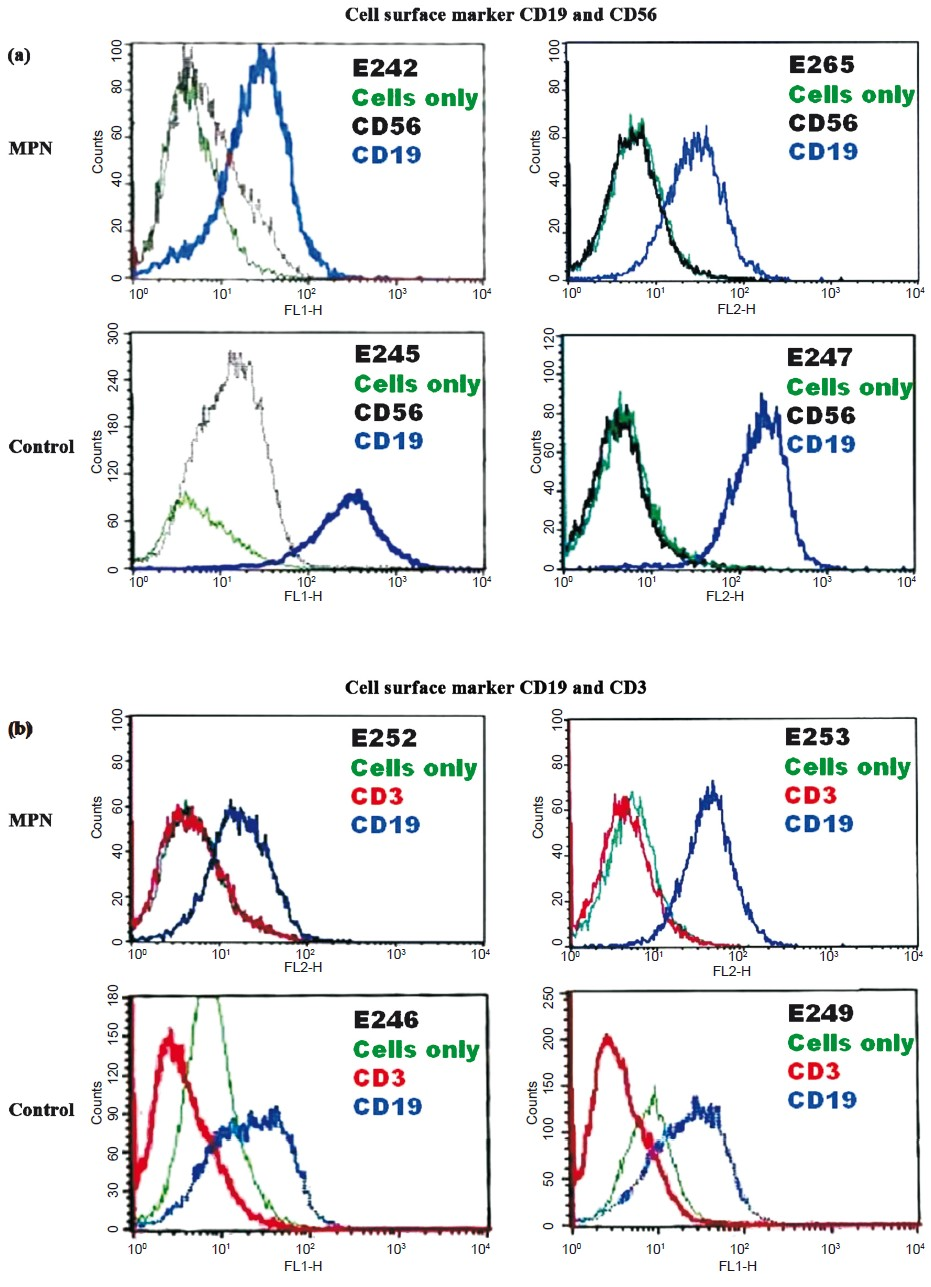
- Flow cytometry analysis for cell surface markers - CD3 (T cell), CD19 (B cell) and CD56 (NK cell) in representative cell lines. Unstained cells were used as internal control. X-axis represents the fluorescence intensity. A forward shift in the peak, caused by binding of fluorophore tagged antibody, as compared to unstained cell is considered positive (a) LCLs E242, E265, E245 and E247 were positive for CD19 and negative for CD56 marker while (b) LCLs E252, E253, E246 and E249 were positive for CD19 and negative for CD3 marker.
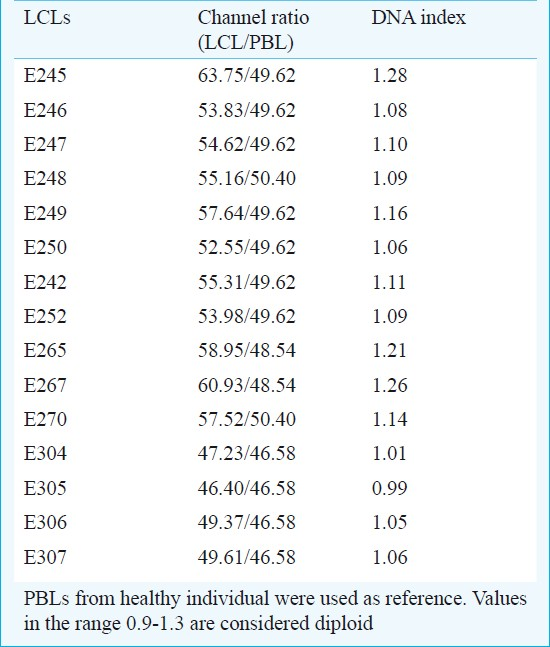
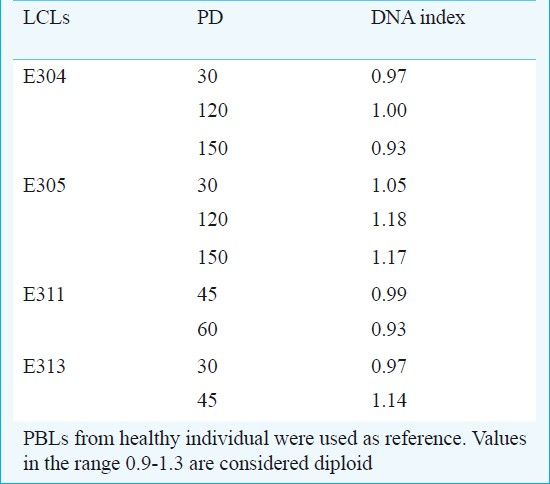
DNA ploidy analysis: DNA ploidy status of the LCLs was assessed immediately after cell line preparation at low population doubling (<5 PD) using PBL from healthy individuals as diploid control. A shift in the position of the diploid peak of LCL away from the expected diploid position of the control can be taken as an evidence of DNA aneuploidy. All the cell lines studied had DI values ranging between 0.8-1.3 and were considered diploid (Table II, Fig. 4). Cell lines in continuous culture can show aberrant DNA content hence ploidy was measured even at higher population doublings of 30, 45, 60, 120 and 150 in a few cell lines. The DI values ranged between 0.93 and 1.18; hence were considered to be diploid (Table III).
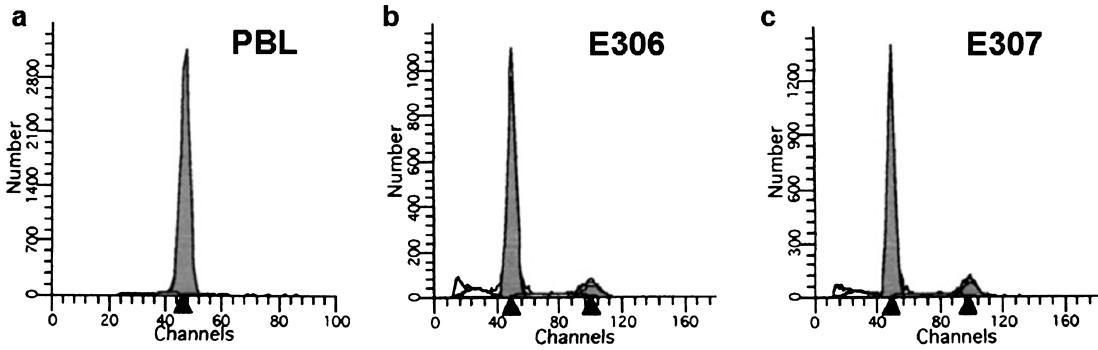
- Flow cytometry analysis showing the diploid status of PBLs isolated from healthy subject and two representative LCLs E306 and E307. The black arrows in the histogram X- axis represent the channel number of the respective cell cycle stage. Arrow at smaller channel number corresponds to G0/G1 stage of cell cycle while the other arrow corresponds to G2/M stage which is located at approximately double position.
Expression and activity of ATM gene: Lytic cycle of EBV elicits a cellular DNA damage response resulting in activation of the ataxia telangiectasia-mutated (ATM) signal transduction pathway21; hence ATM expression in the cell lines and their respective PBL was studied. There was apparently no change in the expression level of ATM gene between the LCL and respective PBL from the subject (Fig. 5). The number of pATM Ser1981 foci seen as distinct nuclear foci in response to cellular DNA damage, were similar in both - the cell line as well as lymphocytes (data not shown).
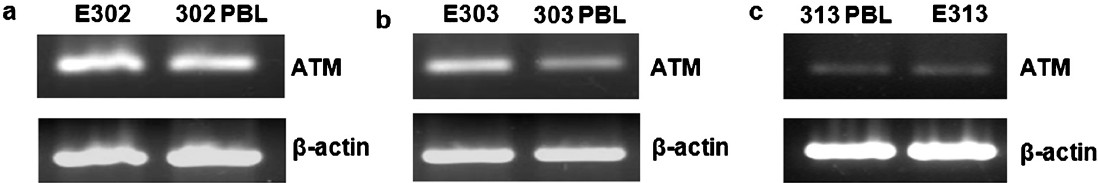
- Expression of ATM gene in different cell lines and their respective PBLs (a) E302 (b) E303 (c) E313. RT-PCR products were run in 2 per cent agarose gel stained with ethidium bromide. β-actin gene was taken as loading control.
Discussion
We report here generation and characterization of LCLs from MPN patients and healthy individuals. LCLs can be generated efficiently as continuously growing cells from an individual following infection of PBLs with EBV-containing supernatants. Such EBV immortalized cells are derived from B lymphocytes and, unlike cell lines derived from other tissues, remain near diploid in nature. Such LCLs can be used for various in vitro studies as well as serve as a source of DNA for genomic studies. Generation of EBV transformed cell lines has proven to be cost-effective, rapid and reliable with minimal deviation from the normal phenotype and genotype.
B95-8 cells derived from Marmoset lymphocytes, infected and immortalized with EBV, are typically used for virus production since these spontaneously produce the B95-8 strain of EBV22. Average time taken for LCL establishment was 3-4 wk extending up to 5 wk in some cases. Variation in the time taken for cell line establishment and growth rate can be attributed to difference in the transformation efficiency occurring due to possible batch-wise difference in the viral titre. Once the cell lines were established, these behaved similarly showing comparable morphology, doubling time, genotypic and cell surface characters and behaviour in phenotypic assays.
Successful transformation of B cells by EBV resulted in enlargement in size and development of aggregates of proliferative cells. Due to the acquired property of cell aggregation LCLs grew as clumps in suspension cultures, with a mean population doubling time of 24 h and when seeded at a density of 0.5-1X106/ml needed to be split twice every week. Early passage cells were cryopreserved immediately after transformation. Established LCLs were cryopreserved at later passages as well, but not later than 30 population doublings.
EBV transformed LCLs are known to exist in two distinguishable forms pre-immortal and post-immortal. In the pre-immortal stage cells proliferate actively and maintain diploid karyotype. These cells are non tumorigenic and die before reaching 160 population doublings. On the other hand, in the post immortalization stage, EBV transformed cells develop a strong telomerase activity and are aneuploid. These also show cellular changes, gene mutations and have the ability to grow indefinitely9. Hence, in the present study, for phenotypic assays LCLs were used within 45-60 PD.
Morphological analysis of cell lines by flow cytometry showed two distinct populations although as EBV is known to specifically transform B cell, only one population is expected. The R1 population represents B cells, based on cell morphology and granularity, and R2 population could be proliferating T/NK cells due to immune response elicited by EBV infected B cells. Conventionally the immune suppression of T cells in LCLs is done by supplementing LCL cultures with cyclosporine-A post infection to improve immortalization8. However, no cyclosporine A was added in the present study. To study the nature of cells in both clusters immunophenotyping was done using CD19, CD3 and CD56 antibodies in a few representative cell lines. All the cell lines tested were positive for B cell marker in both the clusters (data not shown). The possible reason for occurrence of R2 population could be differential size and granularity of the cells arising due to spontaneous differentiation into smaller lymphoid form with shrunken nucleus. These cells in usual cell culture further undergo apoptosis20.
Cell immortalization and proliferation in continuous cultures can result in aberrant DNA changes like aneuploidy or tetraploidy. Hence ploidy status of the cell lines was ascertained at both, low and high population doublings ranging from <5 to >100 PD taking normal PBL as control. DNA index (DI) ratio was calculated to establish the ploidy status. DI ratio of 1 was considered as diploid23 while DI values ranging from 1.9-2.1 with proportion of cells greater than the G2/M fraction of normal control, after correction of the aggregates were considered as tetraploid24. All the cell lines studied at lower (<5 PD) as well as higher population doubling (>100 PD) had a DI ratio ranging between 0.9-1.3 and were considered to be diploid.
As described in earlier reports EBV remains episomal in lymphoblastoid cell lines maintaining a latent infection, although there is a small subpopulation of cells that switches spontaneously from a latent stage of infection into the lytic cycle. Induction of EBV lytic replication elicits cellular DNA damage response dependent on ATM19. DNA damage sensor MRN (Mre II, Rad 50 and Nbsl) complex and phosphorylated ATM are recruited to viral replication compartments, presumably recognizing newly synthesized viral DNAs as abnormal DNA structures21. The LCLs in the present study were established with an aim to eventually study the contribution of DNA damage repair in vitro in UADT MPN patients and to elucidate the mechanism involved. Hence it was necessary to ensure that the process of EBV transformation did not affect expression and activity of DNA repair gene ATM. It was observed that EBV transformation did not elicit DNA repair pathway dependent on ATM as there were no pATM foci seen in LCLs (data not shown). This was further confirmed by measuring γH2AX foci in cell lines which was also found to be negative (data not shown). Also there was no change in ATM gene expression in cell lines and PBLs as revealed by semi-quantitative RT PCR data.
This property of LCLs to be able to grow in continuous culture together with maintaining a close similarity to the parent lymphocytes has been exploited in various studies. There are numerous reports where LCLs have been used as a source of basic biomolecules like, DNA, including mitochondrial DNA, RNA and protein9. DNA isolated from LCLs has been widely used for mutation analysis2526, while RNA isolated from these cell lines has been commonly used for cDNA library preparation and to assess transcriptional response to genotoxins using high throughput technologies including cDNA microarray27, together with this LCLs have as well been used for proteomic studies28.
For large scale population based studies, LCLs provide a constant supply of starting material for a variety of assays, sparing the need of re-sampling. LCLs have been established as an excellent model system not only in basic biomedical studies but also to carry out genomic wide high throughput research thus showing their utility in a broad range of biomedical research2930. All this emphasizes the research utility of LCLs as a surrogate for isolated lymphocytes. The cell lines developed in the present study have been well characterized and provide a valuable, cost effective, in vitro model system for genotypic and phenotypic assays ensuring adequate starting material for current and future analysis.
Acknowledgment
ACTREC intramural funding for the study and CSIR Senior Research Fellowship to the first author (TH) is gratefully acknowledged.
References
- Glutathione S-transferase M1 or T1 null genotype as a risk factor for developing multiple primary neoplasms in the upper aero-digestive tract, in Indian males using tobacco. Oral Oncol. 2004;40:84-91.
- [Google Scholar]
- Genotype, phenotype and cancer: role of low penetrance genes and environment in tumour susceptibility. J Biosci. 2005;30:93-102.
- [Google Scholar]
- The impact of genetic factors on the incidence of multiple primary tumors (MPT) of the head and neck. Cancer Lett. 2005;224:263-78.
- [Google Scholar]
- Relation between DNA repair, apoptosis and chromosomal aberrations in presence of pifithrin-alpha, an inhibitor of p53. Mutat Res. 2010;701:92-7.
- [Google Scholar]
- Mutagen sensitivity in oral cancer patients, healthy tobacco chewers and controls. Acta Cytol. 2010;54:169-74.
- [Google Scholar]
- Thalidomide induces phosphorylation of histone H2AX and increases rate of apoptosis caused by fludarabine in malignant lymphocytes of chronic lymphocytic leukemia in short-term cell cultures. Leuk Res. 2009;33:997-1000.
- [Google Scholar]
- A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet. 1986;73:320-6.
- [Google Scholar]
- Characterization of publicly available lymphoblastoid cell lines for disease-associated mutations in 11 genes. Clin Chem. 2005;51:2156-9.
- [Google Scholar]
- Genetic variation predicting cisplatin cytotoxicity associated with overall survival in lung cancer patients receiving platinum-based chemotherapy. Clin Cancer Res. 2011;17:5801-11.
- [Google Scholar]
- Steps involved in immortalization and tumorigenesis in human B-lymphoblastoid cell lines transformed by Epstein-Barr virus. Cancer Res. 2004;64:3361-4.
- [Google Scholar]
- DNA repair capacity of lymphoblastoid cell lines from sisters discordant for breast cancer. J Natl Cancer Inst. 2005;97:127-32.
- [Google Scholar]
- Removal of benzo(a)pyrene diol epoxide (BPDE)-DNA adducts as a measure of DNA repair capacity in lymphoblastoid cell lines from sisters discordant for breast cancer. Environ Mol Mutagen. 2002;40:93-100.
- [Google Scholar]
- DNA repair in lymphoblastoid cell lines from patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1999;125:185-90.
- [Google Scholar]
- Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323:795-801.
- [Google Scholar]
- Case-control study and meta-analysis of SULT1A1 Arg213His polymorphism for gene, ethnicity and environment interaction for cancer risk. Br J Cancer. 2008;99:1340-7.
- [Google Scholar]
- Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988;239:487-91.
- [Google Scholar]
- Selected methods in cellular immunology. San Francisco: Freeman and Co; 1980. p. :16-9.
- Innate apoptosis of human B lymphoblasts transformed by Epstein-Barr virus: modulation by cellular immortalization and senescence. Cell Struct Funct. 2003;28:61-70.
- [Google Scholar]
- Epstein-Barr virus lytic replication elicits ATM checkpoint signal transduction while providing an S-phase-like cellular environment. J Biol Chem. 2005;280:8156-63.
- [Google Scholar]
- Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci USA. 1972;69:383-7.
- [Google Scholar]
- Guidelines for implementation of clinical DNA cytometry. International Society for Analytical Cytology. Cytometry. 1993;14:472-7.
- [Google Scholar]
- Identification of new minimally lost regions on 18q in head and neck squamous cell carcinoma. Cancer Res. 2000;60:3397-403.
- [Google Scholar]
- Characterization of ATM gene mutations in 66 ataxia telangiectasia families. Hum Mol Genet. 1999;8:69-79.
- [Google Scholar]
- Microarray analysis of bleomycin-exposed lymphoblastoid cells for identifying cancer susceptibility genes. Mol Cancer Res. 2006;4:71-7.
- [Google Scholar]
- Human lymphoblastoid proteome analysis reveals a role for the inhibitor of acetyltransferases complex in DNA double-strand break response. Cancer Res. 2006;66:1473-80.
- [Google Scholar]
- Genome-wide discovery of loci influencing chemotherapy cytotoxicity. Proc Natl Acad Sci USA. 2004;101:11809-14.
- [Google Scholar]
- Mycophenolic acid response biomarkers: a cell line model system-based genome-wide screen. Int Immunopharmacol. 2011;11:1057-64.
- [Google Scholar]






