Translate this page into:
Current tuberculosis diagnostic tools & role of urease breath test
Reprint requests: Dr William R. Bishai, Co-director Center for Tuberculosis Research, Department of Medicine/Division of Infectious Diseases, Johns Hopkins School of Medicine, 1550 Orleans Street Suite 103, Baltimore, MD 21231-1001, USA e-mail: wbishai@jhmi.edu
-
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Tuberculosis (TB) remains a significant public health issue worldwide especially in developing countries, where the disease is endemic, and effective TB diagnostic as well as treatment-monitoring tools are serious barriers to defeating the disease. Detection of pathogen-specific metabolic pathways offers a potential alternative to current methods, which focus on bacterial growth, bacterial nucleic acid amplification, or detection of host immune response to the pathogen. Metabolic pathway detection may provide rapid and effective new tools for TB that can improve TB diagnostics for children and HIV infected patients. Metabolic breath tests are attractive because these are safe, and provide an opportunity for rapid point of care diagnostics and tool for drug efficacy evaluation during clinical trials. Our group has developed a rabbit urease breath test model to evaluate the sensitivity and the specificity of urease based detection of Mycobacterium tuberculosis. TB infected rabbits were given stable isotopically labelled urea as the substrate. The urea tracer was metabolized to 13C-CO2 and detected in exhaled breaths using portable infrared spectrometers. The signal correlated with bacterial load both for primary diagnostics and treatment monitoring. Clinical trials are currently ongoing to evaluate the value of the test in clinical management settings. Urea breath testing may provide a useful diagnostic and biomarker assay for tuberculosis and treatment response.
Keywords
Biomarker
TB diagnostic and treatment monitoring
UBT
Introduction
About two billion people (one-third of the world's population) carry latent tuberculosis (TB) infection and more than nine million of them become sick each year with active TB, which can be spread to others. It disproportionately affects people in resource-poor settings, particularly those in Asia and Africa12. More than 90 per cent of new TB cases and deaths occur in those parts of the world, posing significant challenges to the livelihood of individuals and developing economies, as TB primarily affects people during their most productive years12. Poor health systems, limited laboratory capacity for case detection, treatment barriers and complications (unreliable drug supply, patients not completing treatment, or prescribing errors), TB and HIV co-infection, and the emergence of drug-resistance34 make TB a major challenge facing public health programmes, particularly in the 22 countries with the highest TB burden (Fig. 1). In addition, TB is difficult to diagnose and its diagnosis is more difficult in individuals with TB/HIV co-infection. The current diagnostic techniques are either inadequate to detect TB cases with precision, or are time consuming, expensive, and require highly equipped laboratories which are not available in developing countries, where the disease is endemic. In this paper, we reviewed the current available diagnostic tools for TB and the potential role of urease breath test (UBT) to both diagnose TB and monitor treatment response.
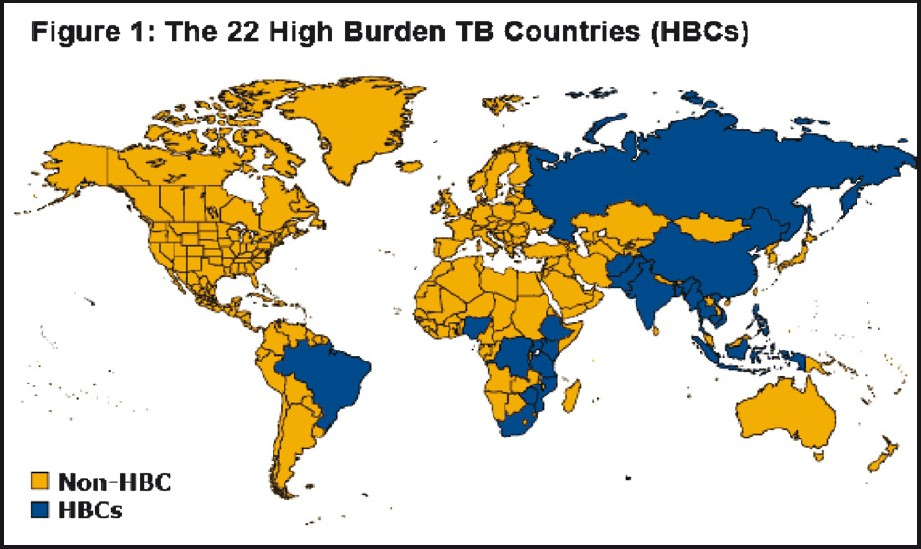
- The 22 high burden TB countries in the word (Source: Kaiser Family Foundation, www.GlobalHealthFacts.org, based on WHO, Global tuberculosis control 2010, Slide Date: March 04, 2011; reprinted with permission from the Henry J. Kaiser Family Foundation, California, USA).
Current TB diagnostic tools
Sputum smear is used to detect acid fast bacilli (AFB) in clinical specimens by Ziehl-Neelsen (Z-N) or fluorescent staining. It is a cost-effective tool for diagnosing patients with TB and to monitor the progress of treatment especially in developing countries. However, there are many drawbacks such as the difficulty of obtaining the sputum sample especially in children and the low sensitivity especially in HIV-infected individuals with AFB smear positivity ranging from 31 to 90 per cent5.
Sputum culture is the gold standard for TB diagnosis with an excellent sensitivity and specificity. The traditional method of inoculating solid medium such as Lowerstein-Jensen (L-J) or 7H10/7H11 media is slow and takes 6-8 wk of incubation to diagnose the infection and further more time to determine the susceptibility patterns. That results in delay in initiation of appropriate therapy.
The introduction of the BACTEC® TB 460 radiometric system (Becton Dickinson, Sparks, MD, USA) in the 1980s allowed the detection of Mycobacterium tuberculosis in a few days as compared with the conventional culture media. The introduction of these rapid and automated systems have increased the sensitivity of isolation of Mycobacterium from clinical samples and has brought down the time required for positive culture substantially (9-10 days). Faster culture results in M. tuberculosis infected patients can result in evidence-based therapy and faster implementation of the planned treatment6. Although a combination of traditional solid and liquid media is recommended and helpful for the primary isolation of Mycobacterium, the process still requires several days and expensive infrastructure.
The microscopic observation drug susceptibility (MODS) assay relies on light microscopy to visualize the characteristic cording morphology of M. tuberculosis in liquid culture. MODS has shorter time to culture positivity (average 8 days) compared with LJ medium (average ~26 days). The cost-effectiveness of MODS (~US $2/ 102/- per sample) is favourable compared with L-J culture (~ US $6/
102/- per sample) is favourable compared with L-J culture (~ US $6/ 307/- per sample)7. Hence, MODS appears to be a promising, novel and inexpensive tool that can rapidly detect TB and drug resistance directly from sputum samples. However, the effectiveness of MODS in HIV/TB case detection, which is a very common co-infection in developing countries, is not yet clearly established. Also the test is only validated for isoniazid and rifampicin susceptibility testing, excluding the other first lines and second lines TB therapy.
307/- per sample)7. Hence, MODS appears to be a promising, novel and inexpensive tool that can rapidly detect TB and drug resistance directly from sputum samples. However, the effectiveness of MODS in HIV/TB case detection, which is a very common co-infection in developing countries, is not yet clearly established. Also the test is only validated for isoniazid and rifampicin susceptibility testing, excluding the other first lines and second lines TB therapy.
The Gen-Probe assay specific for M. tuberculosis complex is a rapid detection, nucleic acid amplification (NAA) test, where results can be obtained as fast as in two hours (provided if a positive culture is present); it also has a high sensitivity of 99 per cent and specificity of 99.2 per cent8. It holds the disadvantage of needing of positive culture that can take several days.
Spoligotyping is a technique for concurrent detection and typing of M. tuberculosis complex bacteria9. This method relies upon amplification of a highly polymorphic direct repeat locus in the M. tuberculosis genome and results can be obtained within one day. Thus, the clinical usefulness of spoligotyping is determined by its rapidity, both in identifying causative bacteria and in providing molecular epidemiologic information on strains. Spoligotyping holds drawbacks of being expensive, and requiring well-equipped laboratory and skilled laboratory personnel, which are not always available in endemic areas.
Line-probe assays (INNO-LiPA® Rif. TB and HAIN Genotype MTBDR plus) and the microfluidic PCR device (GenExpert®/Cepheid) allow both rapid detection of TB and also drugs resistance patterns1011. The INNO-LiPA® Rif. TB can simultaneously detect M. tuberculosis and the presence of a mutation in the rpoB gene, which confers resistance to rifampicin with a sensitivity of 95 per cent and specificity nearly 100 per cent when isolates from culture were used1213. However, INNO-LiPA® Rif. TB is less accurate when directly applied on clinical sputum specimens12. The new Hain “GenoType® MTBDR plus” test can be used both on culture-based isolates and directly on smear positive sputum samples from patients with pulmonary TB. The MTBDR assay has the advantage of identifying mutations in the rpoB gene (rifampicin resistance) as well as mutations in the katG gene (high-level of isoniazid resistance) and inhA gene (low-level of isoniazid resistance). The MTBDR assay can detect at least 90 per cent of MDR-TB cases in a few hours13. GeneXpert® MTB/ RIF uses heminested real-time polymerase chain reaction (PCR) assay to amplify M. tuberculosis specific sequence of the rpoB gene, which is probed with molecular beacons for mutations within the rifampin-resistance determining region1415. The sensitivity of a single direct MTB/RIF test for culture-confirmed tuberculosis was 92.2 per cent and rose to 96.0 per cent with the additional testing of a pelleted sample16. The MTB/RIF test provided sensitive detection of tuberculosis and rifampicin resistance directly from untreated sputum in less than 2 h with minimal hands-on time16.
Such novel technologies have been criticized for being expensive and excluding many drugs for the resistance patterns profile.
Urease activity of M. tuberculosis and applications
The M. tuberculosis urease enzyme encoded by ureA, ureB and ureC (Rv1848, Rv1849 and Rv1850) hydrolyzes urea into carbon dioxide and ammonia17. The M. tuberculosis urease is a likely bacterial virulence factor, which may subvert the host acidification of the phagosome microenvironment. Urease activity has been used in the past as a key test in the identification of microorganisms18. Sohngen reported urease activity in mycobacteria in 1913 (as noted by Urabe and Saito)19. With the classification of mycobacteria in the 1950s by Timpe20 and Runyon21, medical and taxonomic interest in mycobacteriology increased. During the following decade, biochemical and morphological findings established mycobacteriology as a distinct area for development22–24 and tests based on urease activity have been consistently utilized for the identification of mycobacterial species25–28. Despite the role of urease testing for mycobacterial species identification, little has been done to employ urease-based tests as point-of-care primary TB diagnostics until 2009, when our group studied the usefulness of rabbit urease breath test for TB diagnosis and treatment monitoring29. An attractive aspect of urease-based diagnostics is that humans lack urease enzymes. Hence, the detection of urease enzyme activity in humans signifies the presence of microbial pathogen or commensal which is urease positive.
Metabolomic application of Helicobacter pylori urease activity
As M. tuberculosis, H. pylori also possesses urease activity to promote its survival. Gastric urease detection has been classically utilized for rapid and non-invasive diagnostics of H. pylori infection30. This assay introduced during 1980s, uses an oral dose of labelled 13C-urea with sampling of the ratio of 13CO2 to 12CO2 in exhaled air after the urea is hydrolyzed in the stomach by the H. pylori urease (Fig. 2). This ratio is readily measured using small portable infrared spectrometers, and the entire procedure can be completed within 30 min31. The test called H. pylori urease breath test (UBT-HP) received United States FDA approval in 1993 and is currently popular for point-of-care diagnosis of H. pylori active infection and its post-treatment eradication. This success of the H. pylori urease breath test comprises the basis of developing a similar breath test for TB.
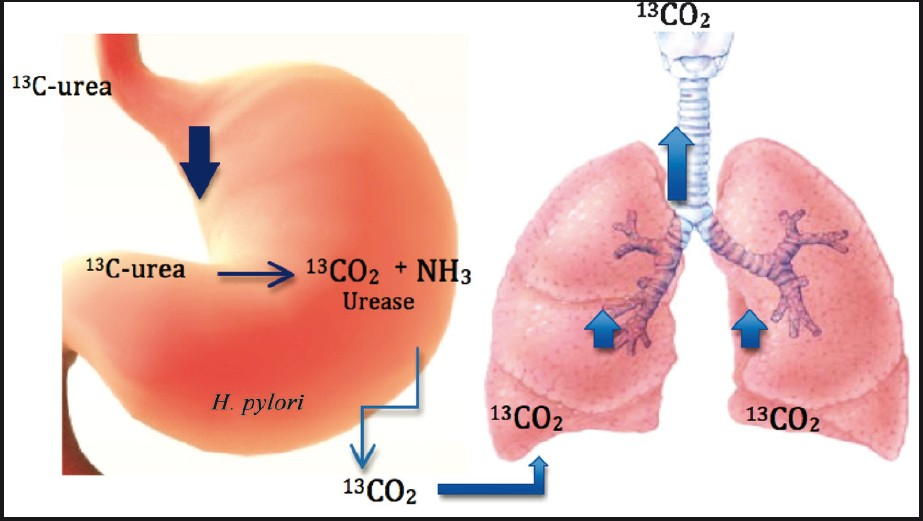
- Labelled urea is degraded by H. pylori in the stomach whereby the labelled CO2 produced is then exhaled and detected in the lungs. The detection of the labelled CO2 implies infection with H. pylori.
Rabbit model of TB-urease breath test (UBT-TB)
Our approach has been to develop a breath test for use in TB similar to the rapid diagnostic for H. pylori infection and as a biomarker of its eradication by drug treatment30. We delivered 13C-urea into lungs of rabbits infected with M. bovis or M. tuberculosis (both of which are urease producing mycobacteria) via bronchoscopic infection, and estimated lung burdens of disease and response to therapy by comparing the UBT results over time29. These rabbits converted 13C-labelled urea into 13CO2 in a matter of minutes (Fig. 3). The 13CO2 in exhaled breath was rapidly detectable with an isotopic ratio mass spectrometer or a small infrared absorbance spectrometer. The UBT signal in rabbits declines as the rabbits are treated with antimicrobial and increases again after therapy is halted29. In contrast to oral UBT-HP, our experiments with rabbit UBT implemented direct instillation of 13C-urea substrate into the lungs or by intravenous injection to increase the specificity for M. tuberculosis. The rabbit UBT model has been limited by the fact that exhaled air is difficult to obtain and animals need to be anesthetized (Fig. 4).
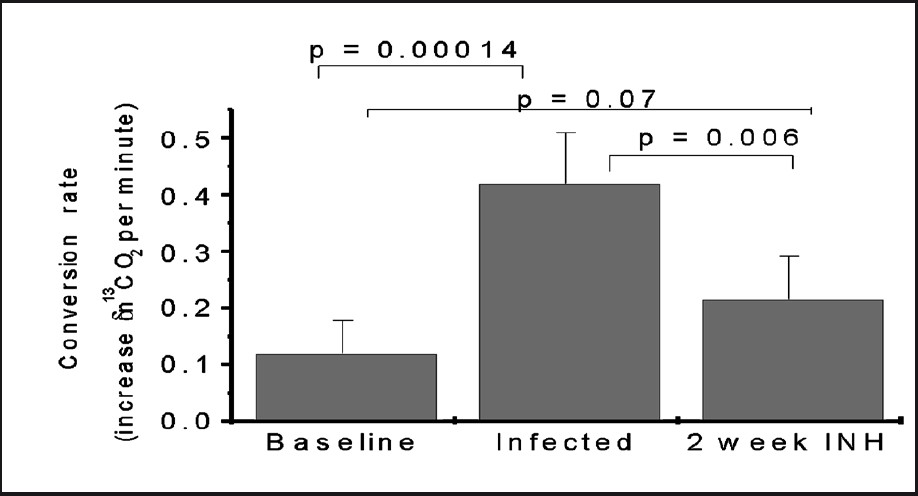
- UBT signal increased in rabbits infected by M. tuberculosis and declined significantly in response to partial TB therapy. Source: Ref 29 (Reprinted with permission).
- Materials to get exhaled air breath from anaesthetized rabbits and transfer into Breathek™ air bag.
Potential utilities of UBT-TB for patient management
UBT provides the possibility of using exhaled breath samples to diagnose TB. It is attractive because it offers a path towards a novel point-of-care diagnostic for TB and TB treatment response. Point-of-care diagnostics are needed for TB especially in high-burden settings in order to reduce the disease impact by providing the possibility to quickly prescribe the appropriate treatment. Thus the UBT may be able to serve as a rapid indicator of treatment failure as occurs in patients with MDR-TB. Resistance to TB drugs is genotypic and new nucleic acid amplification tests (such as GeneXpert®) offer significant steps forward in rapidly detecting certain forms of resistant clinical isolates. However, a proportion of resistant infections will not be identified molecularly and some molecular resistant genotypes may not follow the phenotype expected32. UBT will have the advantage to follow the in vivo real time impact of the treatment on bacterial death. Furthermore, the conversion rate per minute showed that the test may rapidly and reliably detect pulmonary tuberculosis associated with a variety of pathologic presentations29.
Isotopic gas ratio analysis can be conducted with compact infrared spectrophotometers such as POCone™ or UBiT® (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan). The exhaled breath sample is easily obtained through one-way valve bags and analyzed by such devices. These devices can be modified and filters incorporated for use for TB infected patients (to avoid contaminations).
The UBT-TB test may also provide a novel biomarker for TB drug therapy trials to improve therapeutic strategies and validate novel TB drug and vaccine candidates. If validated, it may provide valuable early information on whether bacterial burdens are responding to antimicrobial therapy. As opposed to currently utilized sputum microbiology analyses being used for drug susceptibility, urea breath testing for M. tuberculosis may allow a rapid assessment of treatment efficacy and the need for treatment modifications for drug resistant TB3334.
UBT, as a metabolomic based test, works on live bacteria. A large cell population of M. tuberculosis during the disease and even during TB treatment is non-replicating and non culturable in normal conditions35. However, UBT has the potential to detect this population and monitor its response to chemotherapy, although the relative expression of urease activity by dormant cells requires investigations.
Specificity of UBT-TB
UBT-TB specificity may be limited by other urease-producing microbial species, such as H. pylori, which is common in high TB incidence areas reaching 70 to 90 per cent of the population36. Interestingly, the specificity may be ameliorated by oral non-absorbable urease inhibitors such as bismuth subsalicylate (commonly used to treat dyspepsia) or proton pump inhibitors (PPIs) by allowing selective suppression of gastrointestinal H. pylori37. TB therapy known to have the potential to suppress H. pylori in the first two weeks also increases the specificity for TB treatment monitoring38.
In addition, specificity may be gained by introducing the 13C-urea tracer via the respiratory tract or intravenously, thereby avoid gastric Helicobacter species.
An additional approach would be to co-administer an unlabelled urea drink not enriched with 13C, but rather has this at normal abundance. This approach would saturate gut urease producers with “cold” tracer and, therefore, any CO2 produced by gut organisms would not be enriched in 13CO2 and thus not confound the signal from the lungs.
Ongoing clinical trials on UBT-TB
In collaboration with partners, our group is investigating the sensitivity and the specificity of oral UBT both for TB diagnostic and treatment response in Mali and South Africa39. Pranactin®-Citric (Breathtek™), an oral granulated powder of 13C-urea approved by United States Food and Drug administration (FDA) for UBT-HP will be used with different time points to assess the best moment to test for pulmonary TB. While the test may be confounded by co-existing gastric H. pylori infection, bismuth subsalicylate will be also evaluated in certain arms of the studies to specifically reduce false-positive signal from H. pylori. The studies will compare UBT-TB to TB standard tests and will include both HIV co-infected and non-HIV TB patients. Tolerability studies upon the nebulized delivery of 13C-urea solution directly to the lungs are also in planning stages.
Conclusion
Despite progress in the last decade in TB diagnostic tool discovery, effort is still needed to have impact on the time and the accuracy of the results. The urea breath test may fulfill the criteria for service as an effective point-of-care diagnostic which requires an easily instituted, cost-effective and rapidly applied technology. UBT may also be valuable for response to therapy and thereby offer early information on the possibility of drugs resistant infections monitoring. Further clinical studies are needed to evaluate the benefits of metabolic breath tests compared to the currently available ones.
Acknowledgment
Authors acknowledge the financial support of Howard Hughes Medical Institute grant (80026555) and US National Institute of Allergy and Infectious Diseases grants (AI30036, AI37856 and AI36973).
References
- WHO. 2009. Tuberculosis Facts, 2009 Update. Available from: http://www.thebody.com/content/art54848.html
- [Google Scholar]
- WHO. 2009. Global Tuberculosis Control 2009, Epidemiology, Strategy, Financing; March 2009; Global Tuberculosis Control 2009, A Short Update to the 2009 report. Available from: http://whqlibdoc.who.int/publications/2009/9789241563802_eng.pdf
- [Google Scholar]
- WHO. 2010. Tuberculosis, Global Laboratory Initiative - fact sheet No. 104. Available from: http://www.who.int/tb/publications/factsheets/en/index.html
- [Google Scholar]
- WHO. 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 Global Report on Surveillance and Response. Available from: http://whqlibdoc.who.int/publications/2010/9789241599191_eng.pdf
- [Google Scholar]
- Negative sputum smear results in HIV-positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tuber Lung Dis. 1993;74:191-4.
- [Google Scholar]
- Diagnosis of tuberculosis in an era of HIV pandemic: a review of current status and future prospects. Indian J Med Microbiol. 2010;28:281-9.
- [Google Scholar]
- Rapid, efficient detection and drug susceptibility testing of Mycobacterium tuberculosis in sputum by microscopic observation of broth cultures. J Clin Microbiol. 2000;38:1203-8.
- [Google Scholar]
- Identification of mycobacteria from culture by using the Gen-Probe Rapid Diagnostic System for Mycobacterium avium complex and Mycobacterium tuberculosis complex. J Clin Microbiol. 1988;26:2120-3.
- [Google Scholar]
- Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907-14.
- [Google Scholar]
- Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med. 2006;355:1539-50.
- [Google Scholar]
- Rapid and sensitive detection of Mycobacterium DNA using Cepheid SmartCycler® and Tube Lysis System. Clin Chem. 2001;47:1917-8.
- [Google Scholar]
- A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis. 2005;5:62.
- [Google Scholar]
- GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J. 2008;32:1165-74.
- [Google Scholar]
- Detection of rifampin resistance in Mycobacterium tuberculosis in a single tube with molecular beacons. J Clin Microbiol. 2001;39:4131-7.
- [Google Scholar]
- Molecular beacon sequence analysis for detecting drug resistance in Mycobacterium tuberculosis. Nat Biotechnol. 1998;16:359-63.
- [Google Scholar]
- Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005-15.
- [Google Scholar]
- Structure of Rv1848 (UreA), the Mycobacterium tuberculosis urease gamma subunit. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:781-6.
- [Google Scholar]
- Reliable urease test for identification of mycobacteria. J Clin Microbiol. 1979;10:134-7.
- [Google Scholar]
- The relationship of “atypical” acid-fast bacteria to human disease: a preliminary report. J Lab Clin Med. 1954;44:202-9.
- [Google Scholar]
- Differential identification of mycobacteria. I. Tests on catalase activity. Am Rev Respir Dis. 1996;94:400-5.
- [Google Scholar]
- A study of nitrate reduction by mycobacteria.The use of the nitratereduction test in the identification of mycobacateria. Acta Tuberc Scand. 1960;48(Suppl):1-119.
- [Google Scholar]
- Classification and identification of mycobacteria. I. Tests employing Tween 80 as substrate. Am Rev Respir Dis. 1964;90:588-97.
- [Google Scholar]
- Identification of mycobacteria by biochemical methods. Bull Int Union Tuberc. 1962;32:13-68.
- [Google Scholar]
- A simple urease test for the classification of mycobacteria. Am Rev Resp Dis. 1961;83:757-61.
- [Google Scholar]
- Simple pyrazinamidase and urease tests for routine identification of mycobacteria. Am Rev Respir Dis. 1974;109:147-51.
- [Google Scholar]
- 13[C]-urea breath test as a novel point-of-care biomarker for tuberculosis treatment and diagnosis. PLoS One. 2010;5:e12451.
- [Google Scholar]
- 14C-urea breath analysis, a non-invasive test for Campylobacter pylori in the stomach. Lancet. 1987;1:1367-8.
- [Google Scholar]
- Meretek POCone Infrared Spectrophotometer. Available from: https://new.fishersci.com/ecomm/servlet/fsproductdetail?LBCID=67669088&catalogId=29104&productId=12873065&langId=-1&storeId=10652&distype=2&isChemical=false&fromSearch=0
- [Google Scholar]
- The in vivo-in vitro paradox in pneumococcal respiratory tract infections. J Antimicrob Chemother. 2002;49:433-6.
- [Google Scholar]
- Tuberculosis in the developing world: recent advances in diagnosis with special consideration of extensively drug-resistant tuberculosis. Curr Opin Infect Dis. 2008;21:454-61.
- [Google Scholar]
- Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229-37.
- [Google Scholar]
- Resuscitation-promoting factors reveal an occult population of tubercle bacilli in sputum. Am J Respir Crit Care Med. 2010;181:174-80.
- [Google Scholar]
- Incidence of Helicobacter felis and the effect of coinfection with Helicobacter pylori on the gastric mucosa in the African population. J Clin Microbiol. 2006;44:1692-6.
- [Google Scholar]
- Inhibition of urease by bismuth (III): Implications for the mechanism of action of bismuth drugs. Biometals. 2006;19:503-11.
- [Google Scholar]
- 14C-urea breath test in patients undergoing anti-tuberculosis therapy. World J Gastroenterol. 2005;11:1712-4.
- [Google Scholar]
- ClinicalTrials.gov Oral urea breath testing for diagnosis and treatment response in pulmonary tuberculosis. Available from: http://clinicaltrials.gov/ct2/show/NCT01301144
- [Google Scholar]






