Translate this page into:
Efficacy & safety of iodopovidone pleurodesis: a systematic review & meta-analysis
Reprint requests: Dr. Ritesh Agarwal, Associate Professor, Department of Pulmonary Medicine, Postgraduate Institute of Medical Education & Research, Sector-12, Chandigarh 160 012, India e-mail: riteshpgi@gmail.com agarwal.ritesh@pgimer.edu.in
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Chemical pleurodesis is an accepted therapy for patients with recurrent pleural effusions and pneumothorax. Iodopovidone has been shown to be safe and effective for chemical pleurodesis in several studies. The aim of this systematic review was to update a previously reported meta-analysis on the efficacy and safety of iodopovidone pleurodesis.
Methods:
Two databases MEDLINE and EMBASE were searched for a period (1952-2010), and studies that have reported success rates with iodopovidone pleurodesis were selected. The proportions with 95 per cent confidence interval (CI) were calculated to assess the outcomes in the individual studies and the results were pooled using a random effects model.
Results:
Thirteen eligible studies with 499 patients were included in the mata-analysis. The success rates varied from 70 to 100 per cent in different studies with the pooled success rate being 88.7 per cent (95% CI, 84.1 to 92.1). The success rate was not affected by the method (tube thoracostomy vs. thoracoscopy, 89.6 vs. 94.2%) or the indication of pleurodesis (pleural effusion vs. pneumothorax, 89.2 vs. 94.9%). The only significant complication reported was chest pain of varying degree. Systemic hypotension was reported in six patients across the studies. There were no deaths associated with iodopovidone pleurodesis. Statistical heterogeneity and publication bias were found.
Interpretation & conclusions:
Iodopovidone may be considered a safe and effective agent for chemical pleurodesis in patients with pleural effusions and recurrent pneumothoraces.
Keywords
Chemical pleurodesis
iodopovidone
pleural effusion
pneumothorax
talc
Pleurodesis is a procedure to achieve symphysis between the two layers of pleura aimed at preventing accumulation of either air or fluid in the pleural space1. The most common indication is malignant pleural effusion. The technique is also used for recurrent pneumothoraces and in selected patients with non malignant pleural effusions. Pleurodesis can be achieved by either a chemical agent or by physical abrasion of the pleural surfaces during thoracotomy or thoracoscopy. An ideal chemical agent for pleurodesis should be highly efficacious, have a high molecular weight and chemical polarity, low regional clearance, rapid systemic clearance, a steep dose-response curve, should be inexpensive and easily accessible, easy to administer, and well tolerated with minimal or no side-effects2. No such agent exists and the search for an ideal agent continues.
There is no global consensus on the currently available best chemical agent for pleurodesis. In a survey from five English-speaking countries (United States, United Kingdom, Canada, Australia, and New Zealand), the most commonly used agent was talc followed by tetracycline derivatives and bleomycin3. Talc is also considered the most effective chemical agent for malignant pleural effusions4, and talc insufflation through thoracoscopy is currently considered to be the best method for chemical pleurodesis especially for spontaneous pneumothoraces. There were serious concerns about the safety of talc with reports of acute respiratory distress syndrome (ARDS) following its administration5, which have been negated subsequently6. The availability and cost of medical grade talc remains a constraint for poor patients in developing countries. Iodopovidone is an inexpensive and widely available topical antiseptic7. It has been shown to be a safe and effective agent for chemical pleurodesis8–11. In an earlier study, we demonstrated the success rate of iodopovidone pleurodesis through tube thoracostomy of 87 and 93 per cent in pleural effusion and pneumothorax, respectively10.
In a meta-analysis in 2006, we demonstrated the efficacy and safety of iodopovidone pleurodesis, however, the conclusions were limited by the small sample size11. Since then, several new studies have been published on iodopovidone pleurodesis. Hence, we conducted an updated systematic review and meta-analysis regarding the current efficacy and safety of chemical pleurodesis with iodopovidone.
Material & Methods
Search strategy: The search was aimed to identify studies that had described the success rates of iodopovidone pleurodesis. For this review, both complete (absence of evidence of fluid reaccumulation on chest radiograph till end of follow up or death) or partial success (reaccumulation of fluid but not requiring therapeutic thoracentesis till end of follow up or death) for pleural effusion was considered as success. For pneumothoraces, absence of recurrence of pneumothorax during follow up was defined as success. To identify studies for inclusion in this review, all the authors independently searched two databases: MEDLINE and EMBASE during 1952-2010 for relevant studies using the following free term: pleurodesis, chemical pleurodesis. The reference lists of primary studies, reviews, and editorials were examined. In addition, we reviewed our personal files. Abstracts, editorials, reviews and case reports, as also studies describing the efficacy of iodopovidone pleurodesis in children were excluded.
Initial review of studies: The initial database created from the electronic searches was compiled, and all duplicate citations were eliminated. Two authors (RA and AK) independently screened these citations, without blinding, by title and abstract to capture the relevant studies. Any disagreement was resolved by discussion between the authors. This database was then screened again to include only primary articles, and the full text of each citation, if available was retrieved; otherwise data were extracted from the abstract. Studies were considered eligible for inclusion if the success rate of iodopovidone pleurodesis in pleural effusions or pneumothorax was reported.
Data abstraction: Data extraction was done on a standard data extraction form. The following items were extracted: (i) publication details (title; authors; and other citation details); (ii) type of study (prospective or retrospective); (iii) aetiology of pleural effusion (malignant or non malignant) and pneumothorax (primary or secondary); (iv) baseline demographics of the study population including age, gender and method used to perform pleurodesis either thoracoscopy or tube thoracostomy; (v) solution type, end-points, duration of follow up and complications of the patients undergoing iodopovidone pleurodesis; (vi) success rates of iodopovidone pleurodesis where the numerator was the number of successful pleurodesis and the denominator was the total number of patients with pleural effusions/pneumothorax; and (vii) complications reported with iodopovidone pleurodesis.
Determination of the pooled effect: The statistical software packages Meta-Analyst (version 3.13; http://tuftscaes.org/meta_analyst/) and StatsDirect [version 2.7.7 for MS Windows; StatsDirect Ltd; Cheshire, UK (http://www.statsdirect.com)] were used for statistical analysis. The success rate was measured by calculating proportion with 95 per cent confidence intervals (CI) for each study and then pooling the data to derive a pooled proportion with 95 per cent CI. The proportions were first turned into a quantity (the Freeman-Tukey variant of the arcsine square root transformed proportion) suitable for the usual fixed and random effects summaries for meta-analysis. The pooled proportion was then calculated as the back-transform of the weighted mean of the transformed proportions, using DerSimonian weights for the random effects model.
Assessment of heterogeneity: The impact of heterogeneity on the pooled estimates of the individual outcomes of the meta-analysis was assessed using the Cochran Q statistic and I2 test12 (measuring the extent of inconsistency among the results of the studies, which was interpreted as the approximate proportion of total variation in study estimates due to heterogeneity rather than sampling error). An I2 value of 40 to 50 per cent indicates significant heterogeneity. As the Cochran Q test has a low sensitivity for detecting heterogeneity, P<0.1 was considered significant for the presence of statistical heterogeneity.
Assessment of publication bias: The presence of publication bias was evaluated using the Begg's funnel plot13, which is a measure of the proportion (in the X-axis) against the standard error of the proportion (in the Y-axis). The publication bias was also estimated using three statistical test14–16: (i) Egger test: which detects funnel plot asymmetry. This is a test for the Y intercept = 0 from a linear regression of normalized effect estimate (estimate divided by its standard error) against precision (reciprocal of the standard error of the estimate); (ii) Harbord's test: similar to Egger's test but uses a modified linear regression method to reduce the false positive rate; and (iii) Begg and Mazumdar's test: tests the interdependence of variance and effect size using rank correlation method.
Results
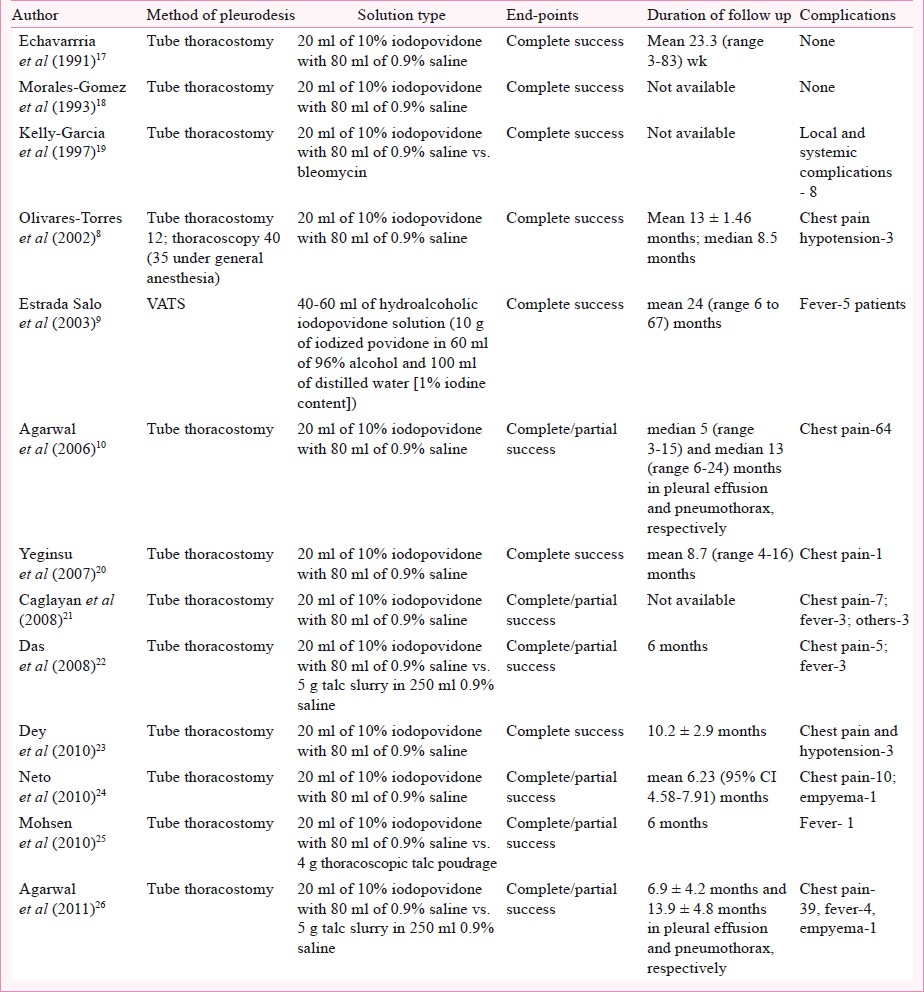
The initial database search retrieved a total of 1,753 citations. Of these, 1,729 studies were excluded as these studies did not involve iodopovidone pleurodesis. Finally, 13 studies that met our inclusion criteria (Tables I and II), and reported the success rates of iodopovidone pleurodesis were included in the final analysis8–1017–26. All the studies were single center except the study by Olivares-Torres et al8. Ten studies were prospective and three were retrospective92024. The studies have included patients with both pleural effusions and pneumothoraces. The pleural effusions were predominantly malignant, and the pneumothorax was spontaneous, both primary and secondary. Tube thoracostomy was the most common method of performing pleurodesis. All studies had used 20 ml of 10 per cent solution of iodopovidone in 80 ml of normal saline except one study9 that used a hydroalcoholic solution of iodopovidone (Table II).
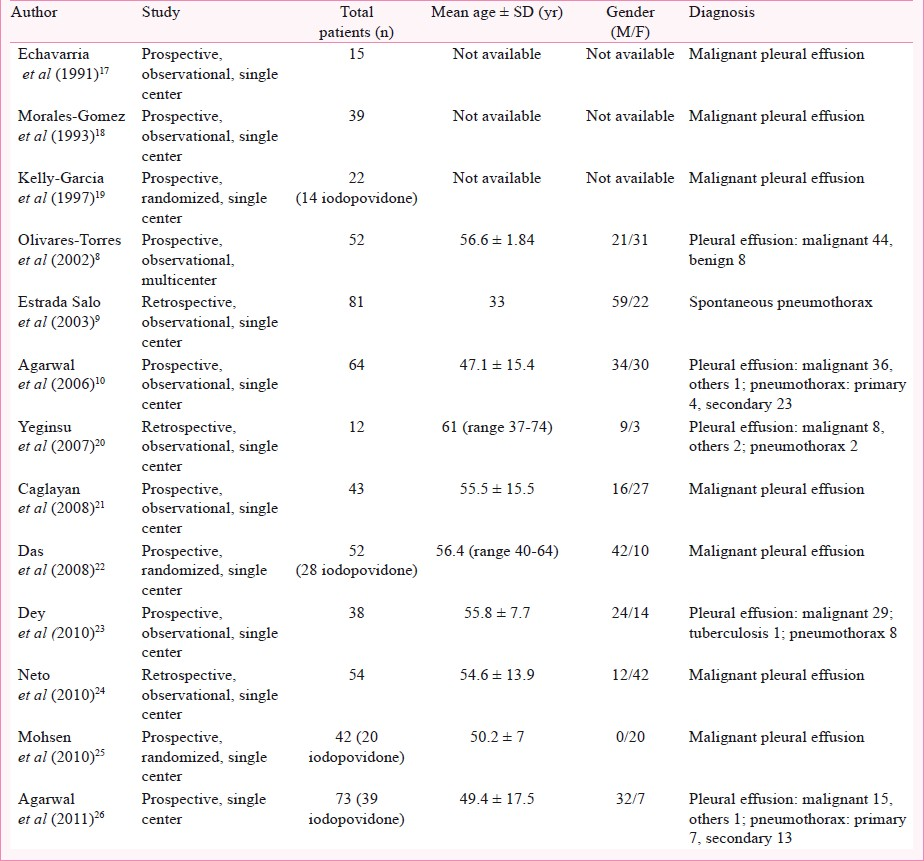
Success rates: The success rate of iodopovidone pleurodesis varied from 70 to 100 per cent in different studies with the pooled success rate being 88.7 per cent (95% CI, 84.1 to 92.1) by the random effects model (Fig. 1). The success rate was not significantly different whether tube thoracostomy (339/378; 89.6%, 95% CI 86.2-94.6) or thoracoscopy (114/121; 94.2%, 95% CI 88.5-94.2) was used for pleurodesis (P=0.13) or whether the aetiology was pleural effusion (322/361; 89.2%, 95% CI 85.5-91.9) or pneumothorax (131/138; 94.9%, 95% CI 89.9-97.5) (P=0.05).
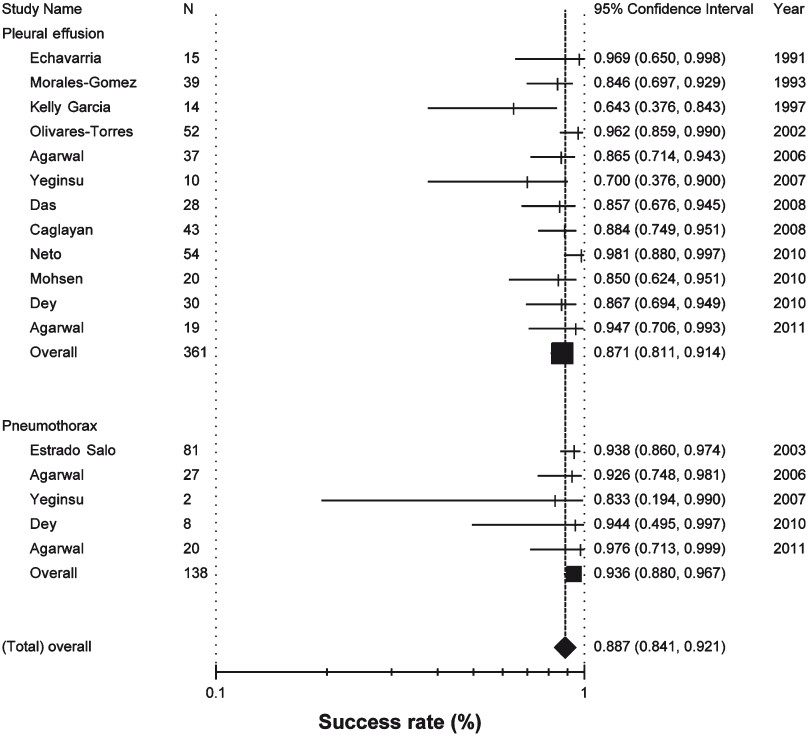
- Success rates of iodopovidone pleurodesis in patients with pleural effusions and pneumothoraces (random effects model). The success rate in the individual studies is represented by a vertical line (percentage) through which runs a horizontal line (95% confidence interval). The square represents the pooled data of the subgroups and the diamond at the bottom represents the pooled success rates across the subgroups.
Complications: There were no deaths or acute respiratory distress syndrome (ARDS) related with iodopovidone pleurodesis. There was no report of visual loss in any of the studies. The reported complications of iodopovidone pleurodesis include chest pain and systemic hypotension (Table II). The occurrence of chest pain has been described to a varying degree by different authors with two studies describing it as a universal occurrence1026. However, only two studies have measured pain systematically where the authors have used a Visual Analog Scale (VAS) for measurement of pain1026.
Heterogeneity: There was significant clinical heterogeneity reflected in the different aetiologies included in the studies (Table I). There was also significant statistical heterogeneity with both I2 (50.8%; 95% CI, 0 to 72.5) and Cochran Q tests (Cochran Q statistic 24.4, P=0.02).
Publication bias: The funnel plots showed evidence of publication bias (Fig. 2). Two of the three statistical tests also showed evidence of publication bias (Begg-Mazumdar: Kendall's tau = -0.44, P=0.03; Egger: bias = -2.28, P=0.009; Harbord-Egger: bias = -2.7, P=0. 08).
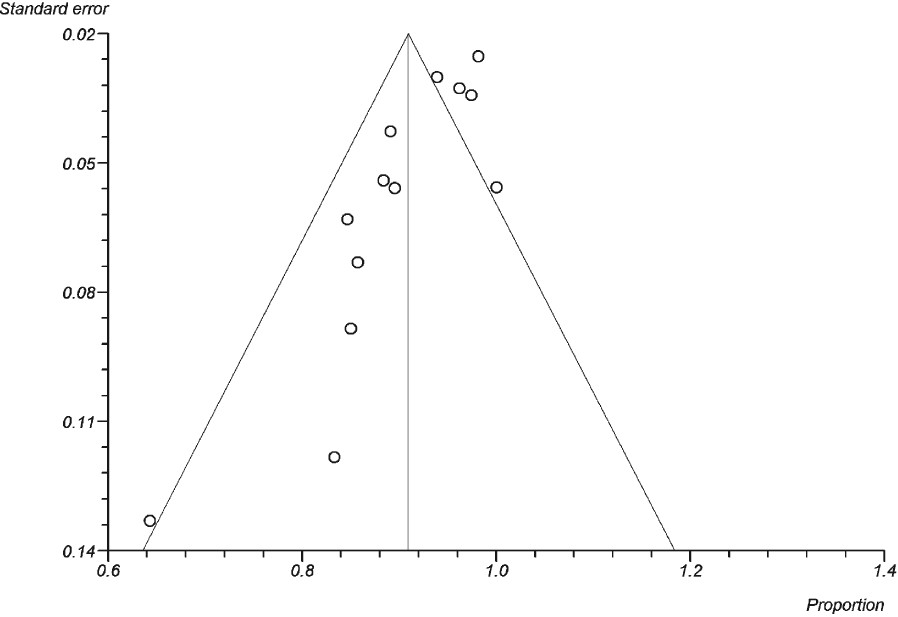
- Funnel plot comparing proportion versus the standard error of proportion for the outcome of iodopovidone pleurodesis. Open circles represent trials included in the meta-analysis. The line in the center indicates the summary proportion. The other lines represent the 95% confidence intervals. Asymmetry about the pooled proportion line is consistent with the presence of publication bias.
Discussion
The results of the present meta-analysis re-affirm that iodopovidone pleurodesis is associated with high success rates, with efficacy rate of 89 and 94 per cent in pleural effusions and pneumothoraces, respectively. There were no serious adverse events including ARDS or deaths associated with the procedure. Chemical pleurodesis is the procedure of choice in the management of recurrent pleural effusions27, and a recognized treatment option in the management of patients with primary or secondary spontaneous pneumothorax28. The question is the choice of the sclerosing agent, which is determined by the efficacy of the agent, its cost, accessibility, safety, ease of administration and the number of administrations needed to achieve a complete response.
In a systematic review that included 1,168 patients, the complete success rate of talc was 93 per cent compared with Corynebacterium parvum (76%), tetracycline (67%), doxycycline (72%) and bleomycin (54%)1. Similarly, among 723 patients drawn from 32 case series of predominantly malignant effusions, talc was completely or partially effective in 91 per cent cases29. In a meta-analysis, talc was found to be the most effective agent for pleurodesis, and thoracoscopic pleurodesis the preferred technique for pleurodesis based on efficacy4. A prospective cohort study with Luzenac talc showed no cases of ARDS following thoracoscopic talc instillation probably due to the large size of the talc used in this study6. However, its limited availability and cost (US $ 45-50) remains a constraint for poor patients in countries with limited resources, and there have even been attempts in using non-pharmaceutical talc for pleurodesis2630.
The use of iodopovidone was first described in 199117. The precise mode of action of iodopovidone remains unclear7. It may be related to the low pH (pH 2.97) of the sclerosing solution, or to the strong oxidative and cytotoxic properties of iodine, which can induce a potent inflammatory response. Theoretically the mechanism could also be similar to that described recently for talc, i.e. production of fibroblast growth factor. It is also probable that iodopovidone may act as a cytotoxic agent on different tumour cell lines in malignant pleural effusions731.
Our earlier systematic review included six studies and 265 patients, wherein we demonstrated the success rate of iodopovidone pleurodesis to be 88.5 per cent for pleural effusions and 93.5 per cent for pneumothorax11. The current systematic review updates the previous meta-analysis by not only including seven additional studies (and total patient number of 499) but also uses advanced meta-analytic techniques which increase the robustness of the results.
The success rate of iodopovidone pleurodesis in this study was 88.7 per cent, which is almost similar to the efficacy of talc pleurodesis, and other inexpensive agents used for chemical pleurodesis which include silver nitrate and quinacrine32. The efficacy of iodopovidone was regardless of the aetiology (pleural effusion vs. pneumothorax) or the technique (tube thoracostomy vs. thoracoscopy) used for performing pleurodesis. Importantly, iodopovidone is not only inexpensive (US$ 1, 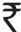 45.00) but is also associated with minimal side effects. The only significant side effect of iodopovidone was the occurrence of chest pain. Only two studies1026 have systematically assessed the occurrence of chest pain by the VAS scale. Hypotension was reported in two studies823 and was found associated with chest pain and is likely to be vasovagal in origin. However, iodine can cause severe allergic reactions, especially in patients with allergic diathesis, and thus one should be prepared to deal with this emergency. Iodine may also precipitate thyrotoxicosis in patients with subclinical hyperthyroidism (Jod-Basedow effect)33. However, in a study with 12 patients, no alterations in thyroid function was noted20. There were no deaths or ARDS associated with this agent. There is also a single report of visual loss associated with iodopovidone pleurodesis using 200-500 ml of 10 per cent iodopovidone34. The recommended dose is 20 ml of 10 per cent iodopovidone with 80 ml of 0.9 per cent saline administered intrapleurally either through tube thoracostomy10 or during thoracoscopy9.
45.00) but is also associated with minimal side effects. The only significant side effect of iodopovidone was the occurrence of chest pain. Only two studies1026 have systematically assessed the occurrence of chest pain by the VAS scale. Hypotension was reported in two studies823 and was found associated with chest pain and is likely to be vasovagal in origin. However, iodine can cause severe allergic reactions, especially in patients with allergic diathesis, and thus one should be prepared to deal with this emergency. Iodine may also precipitate thyrotoxicosis in patients with subclinical hyperthyroidism (Jod-Basedow effect)33. However, in a study with 12 patients, no alterations in thyroid function was noted20. There were no deaths or ARDS associated with this agent. There is also a single report of visual loss associated with iodopovidone pleurodesis using 200-500 ml of 10 per cent iodopovidone34. The recommended dose is 20 ml of 10 per cent iodopovidone with 80 ml of 0.9 per cent saline administered intrapleurally either through tube thoracostomy10 or during thoracoscopy9.
The present meta-analysis had certain limitations. As this is not an individual patient data meta-analysis, the baseline characteristics of patients that could influence pleurodesis success such as pleural fluid pH and performance status, were not known. The other limitation was presence of statistical heterogeneity despite using a random-effects model. Ideally, a meta-analysis should be performed when the individual studies are sufficiently homogeneous in terms of participants, interventions and outcomes. Such a situation is unlikely to occur. Another limitation was the occurrence of publication bias. In our study, it could be due to smaller trials as only one trial was multi-center. Thus, the results of this analysis need to be interpreted with caution keeping these limitations in mind.
In conclusion, with limitations of the analysis, the results support iodopovidone as an effective agent for chemical pleurodesis in patients with pleural effusions and recurrent pneumothoraces.
References
- Chemical pleurodesis for malignant pleural effusions. Ann Intern Med. 1994;120:56-64.
- [Google Scholar]
- BTS guidelines for the management of malignant pleural effusions. Thorax. 2003;58(Suppl 2):ii29-38.
- [Google Scholar]
- Pleurodesis practice for malignant pleural effusions in five English-speaking countries: survey of pulmonologists. Chest. 2003;124:2229-38.
- [Google Scholar]
- Pleurodesis for malignant pleural effusions. Cochrane Database Syst Rev (1):CD002916.
- [Google Scholar]
- Safety of pleurodesis with talc poudrage in malignant pleural effusion: a prospective cohort study. Lancet. 2007;369:1535-9.
- [Google Scholar]
- Iodopovidone: an inexpensive and effective agent for chemical pleurodesis. Lung Cancer. 2007;55:253-4.
- [Google Scholar]
- Spontaneous pneumothorax: pleurodesis with an iodo-povidone hydroalcoholic solution. Arch Bronconeumol. 2003;39:171-4.
- [Google Scholar]
- Efficacy and safety of iodopovidone pleurodesis through tube thoracostomy. Respirology. 2006;11:105-8.
- [Google Scholar]
- Efficacy and safety of iodopovidone in chemical pleurodesis: a meta-analysis of observational studies. Respir Med. 2006;100:2043-7.
- [Google Scholar]
- Analysing and presenting results. In: Alderson P, Green S, Higgins JPT, eds. Cochrane reviewers’ handbook 4.2.2 (updated March 2004). Chichester, UK: John Wiley & Sons Ltd.; 2004. p. :68-139.
- [Google Scholar]
- An approach to assessing publication bias prior to performing a meta-analysis. Stat Sci. 1992;7:237-45.
- [Google Scholar]
- A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443-57.
- [Google Scholar]
- Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-101.
- [Google Scholar]
- Intracavitary treatment of malignant pleural effusion with iodine-povidone. Rev Med Panama. 1991;16:69-74.
- [Google Scholar]
- Pleurodesis con yodopovidona en el derrame pleural neoplásico. Rev Inst Nal Enf Resp Mex. 1993;6:71-4.
- [Google Scholar]
- Iodopovidone and bleomycin pleurodesis for effusions due to malignant epithelial neoplasms. Arch Med Res. 1997;28:583-5.
- [Google Scholar]
- Iodopovidone pleurodesis does not effect thyroid function in normal adults. Interact Cardiovasc Thorac Surg. 2007;6:563-4.
- [Google Scholar]
- Efficacy of iodopovidone pleurodesis and comparison of small-bore catheter versus large-bore chest tube. Ann Surg Oncol. 2008;15:2594-9.
- [Google Scholar]
- A study of comparison of efficacy and safety of talc and povidone iodine for pleurodesis of malignant pleural effusions. J Indian Med Assoc. 2008;106:589-90.
- [Google Scholar]
- Iodopovidone pleurodesis: experience of a tertiary hospital in Kolkata. Singapore Med J. 2010;51:163-5.
- [Google Scholar]
- Efficacy and safety of iodopovidone pleurodesis in malignant pleural effusions. Respirology. 2010;15:115-8.
- [Google Scholar]
- Local iodine pleurodesis versus thoracoscopic talc insufflation in recurrent malignant pleural effusion: a prospective randomized control trial. Eur J Cardiothorac Surg. 2011;40:282.
- [Google Scholar]
- A RCT on the efficacy of cosmetic talc vs.iodopovidone for chemical pleurodesis. Respirology. 2011;16:1064-9.
- [Google Scholar]
- Treatment options for malignant pleural effusion. Curr Opin Pulm Med. 2009;15:380-7.
- [Google Scholar]
- Talc pleurodesis for the treatment of pneumothorax and pleural effusion. Chest. 1994;106:1215-22.
- [Google Scholar]
- Taurolidine and povidone-iodine induce different types of cell death in malignant pleural mesothelioma. Lung Cancer. 2007;56:327-36.
- [Google Scholar]
- Alternative widely available, inexpensive agents for pleurodesis. Curr Opin Pulm Med. 2005;11:340-4.
- [Google Scholar]






