Translate this page into:
Effects of heat stress on endocrine functions & behaviour in the pre-pubertal rat
Reprint requests: Prof. Dr Bayram Yilmaz, Yeditepe University, Faculty of Medicine, Department of Physiology, 34755, Istanbul, Turkey e-mail: bayram2353@yahoo.com
-
Received: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
Heat stress related hyperthermia may cause damage to various organ systems. There are very few studies on the effects of hyperthermia on the endocrine system. We therefore, investigated effects of exogenously induced hyperthermia on adrenal, testicular and thyroid functions and behavioural alterations in pre-pubertal male Sprague-Dawley rats.
Methods:
Three groups of 30-day old rats (n=7 per group) were used. Body temperature was increased to 39°C (Group I) and 41°C (Group II) in a hyperthermia induction chamber for 30 min. The rats in the Group III served as control (36 °C). All animals received saline and were decapitated 48 h after the experiments. Serum free triiodothyronin (fT3), free thyroxine (fT4), total testosterone and dehydroepiandrosterone sulphate (DHEA-S) levels were determined by chemiluminescence assay, and corticosterone by enzyme immunoassay. Testes, pituitary and adrenal glands were dissected out and processed for histopathological examination. To assess activity and anxiety of the animals, the open field test and elevated-0-maze test, respectively, were used in all groups 24 h before (day 29) and after (day 31) hyperthermia induction.
Results:
Serum corticosterone levels (3.22±1.3) were significantly reduced in the 39°C (1.3±0.9) and 41°C (1.09±0.7) hyperthermia groups (P<0.01) compared to controls. Serum levels of thyroid hormones did not significantly differ among the groups. DHEA-S and testosterone values were below the limit of detection in all groups. Histopathological examination revealed that there was mild hydropic degeneration in the pituitary and adrenal glands. Apoptotic germ cells were seen in the seminiferous tubules of pre-pubertal male rats exposed to hyperthermia (41°C). Progression time in the open field test was significantly decreased and anxiety test scores increased in animals exposed to 39°C compared to the control group (P<0.01). These parameters were more pronounced in the 41°C hyperthermia group.
Interpretation & conclusions:
Our results show that heat exposure-induced stress may cause delayed reduction in serum corticosterone levels which may be associated with behavioural deficits in pre-pubertal male rats.
Keywords
Behaviour
corticosterone
endocrine system
heat stress
rat
Hyperthermia may be a consequence of environmental conditions, microbial infections and/or hyperthyroidism. Although regulation of body temperature and individual adaptation to environmental climatic changes is well documented, little is known about mechanisms and pathological aspects of hyperthermia1–3.
Hyperthermia may cause damage in various organs and systems in the body2. However, most of the studies investigating the adverse effects of hyperthermic conditions have focused on the central nervous system4. Blood-brain barrier (BBB) permeability has been shown to be impaired by hyperthermia in experimental models35. Leakage of serum proteins within the brain micro-fluid environment appears to be the main factor for brain oedema formation5. Heat-related neuronal degeneration has also been reported6. It has been shown that hyperthermia increases apoptotic cell death, a condition that is affected by duration of hyperthermia67. Thus, increased brain hyperthermia may cause neurotoxicity either directly or through disruption of BBB.
Hyperthermia is one of the most frequent causes of paediatric complaints leading to hospital admission. Infant and child brain is susceptible to hyperthermia and may undergo various pathological conditions89. There are limited studies on heat-induced alterations in endocrine functions and behavioural dysfunctions, particularly in infants and children3. A few studies demonstrated adverse effects of hyperthermia on the brain in rats10–12. Hyperthermia may impair cognitive functions13, induce problems in coping and behaviour14 including motor functions9. Developing rats exposed to hyperthermia have been shown to display signs of increased anxiety in the elevated-plus maze, but these changes were not associated with increased susceptibility to depression-like behaviour15. Hyperthermia is an important stress factor and known to increase blood cortisol levels16. This is expected since hypothalamo-pituitary-adrenocortical (HPA) axis is activated in response to stressors such as heat and inflammation17. It has been reported that thyroid function may be altered by hyperthermic conditions18–19. There are several reports indicating that increased temperature inhibits spermatogenesis2021. However, post-hyperthermic effects on testicular functions have not been studied in pre-pubertal rats.
In this study, we have examined effects of heat exposure-induced hyperthermia on various endocrine functions and behaviours in pre-pubertal male rats.
Material & Methods
The study was conducted in the Department of Physiology, Yeditepe University, Istanbul, Turkey. Pre-pubertal (30-day old) male Sprague-Dawley rats were used in this study. The animals were obtained from Yeditepe University Medical School Experimental Research Center (YUDETAM) and housed at controlled room temperature (21±1°C) with 12:12 h light:dark cycle. Standard pellet diet and water were provided ad libitum. The rats were divided into three groups (n=7 per group). Body temperature was increased to 39°C (Group I) and 41°C (Group II) by heat exposure in a Hyperthermia Induction Chamber for 30 min. The rats in the Group III served as control (36 °C). The ambient temperature of the laboratory was maintained at 21°C. Hyperthermia Induction Chamber (a large plexyglass box: 40 × 40 × 35 cm) was designed in our laboratory. A thermostat-controlled heater was fitted at the top-lid of the chamber and temperature was continuously monitored by a thermometer throughout the experiment. The animals in each group were placed and exposed to heat stress together (n=7). Core temperature of the animals was monitored by using a rectal thermistor attached to a Harvard Homeothermic System (Kent, UK), throughout the experiments. The body temperature was not allowed to exceed 39 and 41°C in the Groups I and II, respectively. The experiments were approved by the Yeditepe University Ethics Committee on Experimental Animals.
All animals were decapitated 48 h after the experiments (on the day 32) and trunk blood was collected. Blood samples were centrifuged (4°C, 670 g) for 10 min, and serum was separated and stored at -20°C until assayed. Serum free triiodothyronine (fT3), free thyroxine (fT4), dehydroepiandrosterone sulphate (DHEA-S) and total testosterone (TTE) levels were determined by chemiluminescence assay (Roche Diagnostics, France) using Modular E170 analyzer. Serum corticosterone levels were determined by enzyme immunoassay (IDS Ltd, Boldon UK)22. Testes, pituitary and adrenal glands were dissected out, fixed in 10 per cent formalin (buffered with pH 7.2) solution and processed for histopathological examination.
To assess the activity and anxiety of the animals, the open field test23 and elevated-0-maze test23, respectively, were used in all groups 24 h before (on the day 29) and after (on the day 31) hyperthermia induction. These behavioural tests were performed in a blinded fashion23.
Open field test: This test was used to detect spontaneous locomotor activity and exploration behaviour. The open field consists of a round arena (diameter: 150 cm) covered by a white plastic floor, surrounded by a 35-cm high sidewall made of white polypropylene. Each rat was placed in a corner of the field and its behaviour (moving or staying in the same area) recorded for 10 min. Testing was carried out in a temperature, noise and light controlled room.
Elevated O maze: The elevated O maze consists of a round 5.5 cm wide polyvinyl-chloride runway with an outer diameter of 46 cm, which is placed 40 cm above the floor and which detects spontaneous locomotor behaviour and correlates of fear and anxiety24. Two opposing 90° sectors are protected by 16 cm high inner and outer walls made of polyvinyl-chloride (closed sectors). The remaining two 90° sectors are not protected by walls (open sectors). Animals were released in one of the closed sectors and observed for 10 min. The total number of zone entries - as correlate of motor activity - and the time spent in the unprotected sector - as correlate of exploration behaviour, fear and anxiety - were registered whenever the animal moved into a sector with all four paws.
Results (Mean ± SD) were statistically analyzed by using one-way analysis of variance followed by LSD test. P<0.05 was considered statistically significant.
Results
Serum corticosterone levels were significantly (P<0.01) reduced in both hyperthermia groups (Group I: 1.3±0.9 and Group II: 1.09±0.7 μg/dl) compared to control (3.22±1.3 μg/dl). Serum levels of thyroid hormones did not significantly differ among the groups. DHEA-S and TTE values were below the limit of detection in all groups (DHEA-S <0.100 μg/dl and TTE <2.0 ng/dl, respectively) (Table).

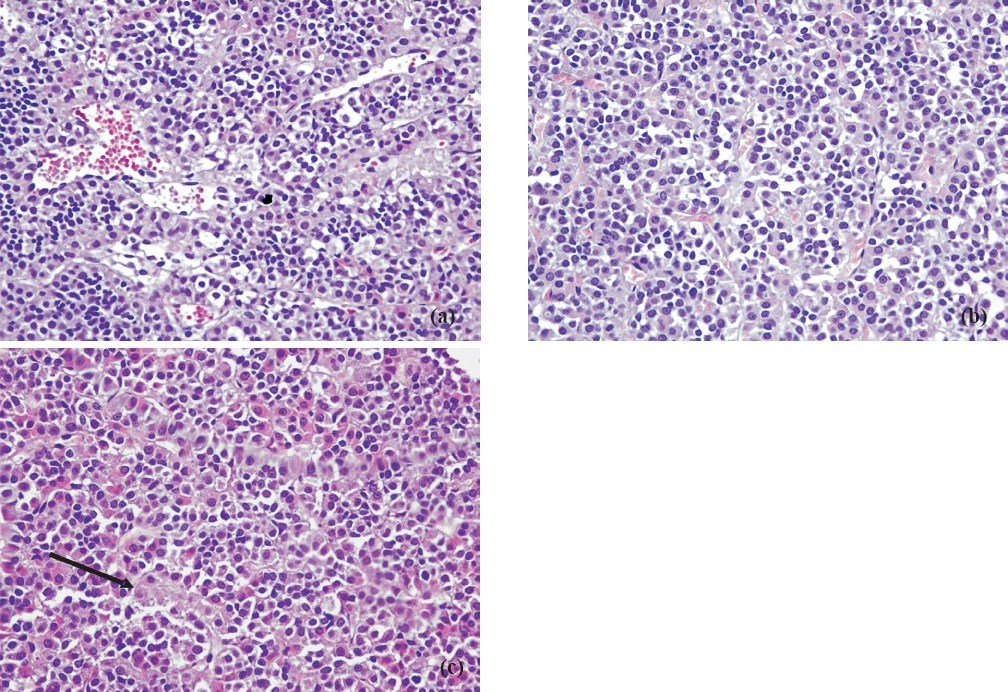
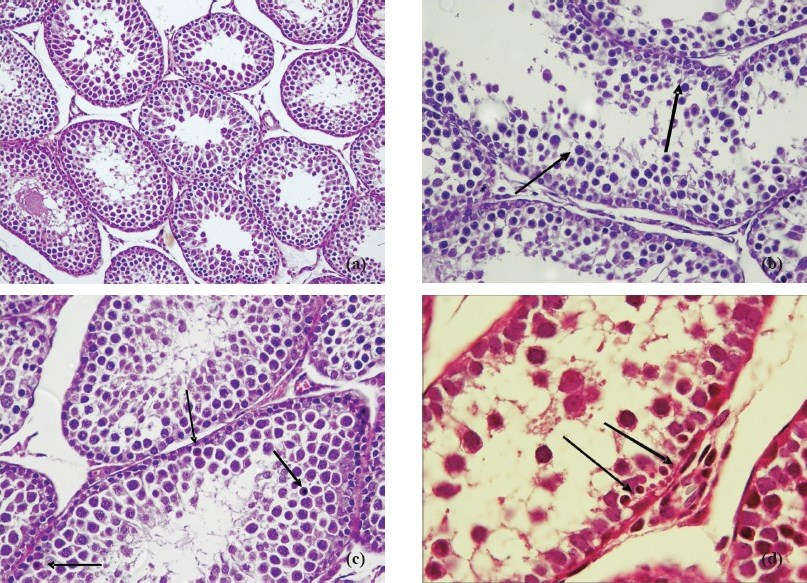
Progression time in the open field test (Fig. 1) was significantly decreased and anxiety test scores (Fig. 2) declined in animals exposed to 39°C compared to the control values (P<0.01). Histopathological findings from the adrenal gland, anterior pituitary and testis are illustrated in Figs 3–5. Histopathological examination revealed that 39°C hyperthermia caused hyperemia in the pituitary and adrenal glands. However, mild degeneration was observed in both glands in the 41°C hyperthermia group. Hydropic swelling was observed in the sperm cells of pre-pubertal rats. Presence of small clear vacuoles was considered to be hydropic degeneration under light microscopic examination. Apoptotic germ cells in the seminiferous tubules of pre-pubertal male rats exposed to hyperthermia (41°C) was also seen. These germ cells appeared to contain small nucleus, high nuclear fragmentation and dark chromatin. In additon, their cytoplasma was hypereosinophilic.
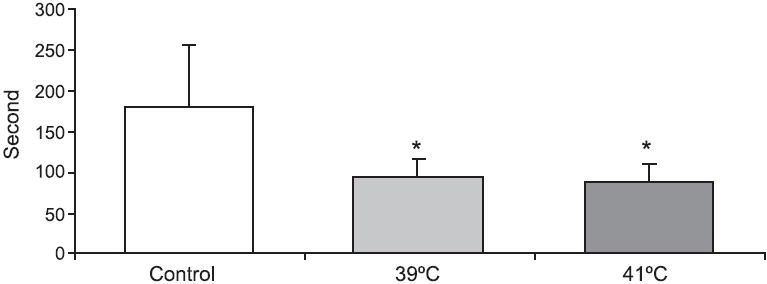
- Animal activity scores (sec) in open field test in pre-pubertal male rats exposed to heat stress (39°C and 41°C hyperthermia) for 30 min. *P<0.05 compared to control group.
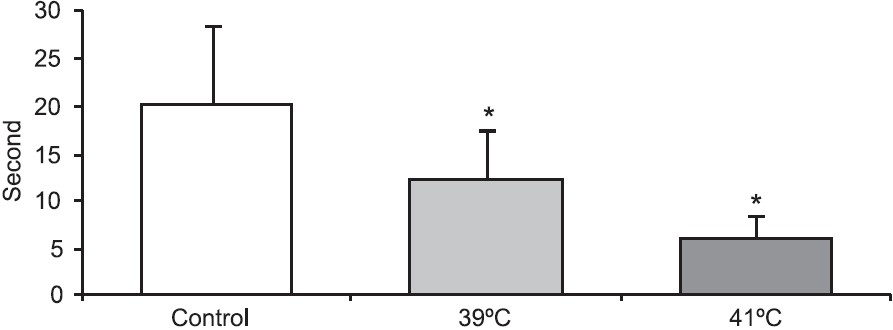
- Anxiety test scores (sec) in pre-pubertal male rats exposed to heat stress (39°C and 41°C hyperthermia) for 30 min. *P<0.05 compared to control group.
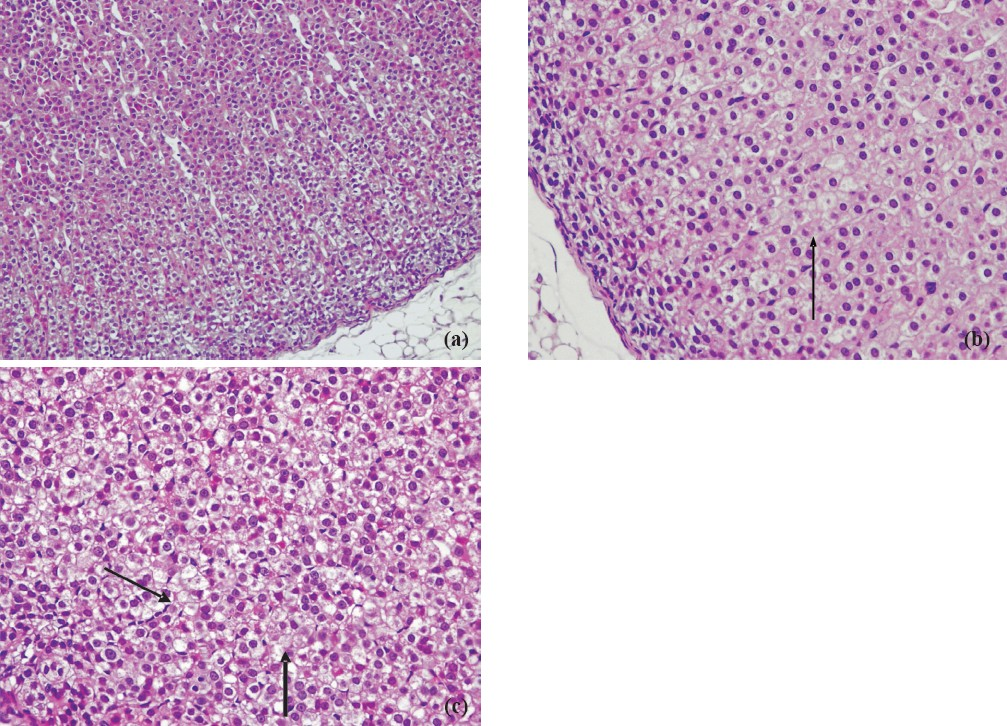
-
a. Adrenal cortex of the control rats. HE × 200. b. Mild hydropic swelling in the adrenal cortex of the 39°C hyperthermia group male rats. HE × 400. c. Mild hydropic degeneration in the adrenal cortex of the 41oC hyperthermia group male rats. HE × 400.
-
a. Control group adenohypophysis. HE × 400. b. Hyperemic adenohypophysis of pre-pubertal male rats exposed to 39° C hyperthermia. HE × 400. c. Hydropic degeneration and focal necrosis (arrow) in the adenohypophysis of pre-pubertal male rats exposed to 41° C hyperthermia. HE × 400.
-
a. Normal maturation of germ cells in the seminiferous tubules in the control group. HE × 200. b. Hydropic swelling in germ cells in the seminiferous tubules of pre-pubertal male rats exposed to 39°C hyperthermia. HE × 400. c. Presence of apoptotic germ cells (arrow) in the seminiferous tubules of pre-pubertal male rats exposed to 41°C hyperthermia. HE × 400. d. Presence of apoptotic germ cells (arrow) in the seminiferous tubules of pre-pubertal male rats exposed to 41°C hyperthermia. HE × 400.
Discussion
It is known that HPA axis is activated in response to various types of stress including heat1525. Acute increases in plasma cortisol levels have been correlated with coping behaviour and adaptation to heat stress26. Rats with impaired HPA axis were less tolerant to heat stress exposure15. In our study, serum levels of corticosterone were significantly lower than the control group. Since the animals were decapitated 48 h after the hyperthermic stress induction, reduced corticosterone may be attributed to post-stress changes, like in post-traumatic stress disorder (PTSD). PTSD is an anxiety disorder that can develop after exposure to a traumatic event27. Previously, plasma corticosterone and adrenocorticotropic hormone (ACTH) levels were found to be low in heat exhausted rats14. In the present study, anxiety scores of the animals in the hyperthermia groups were associated with decreased corticosterone levels. Hyperthermia-related anxiety has been shown in developing rats28. Our findings show such anxiety behaviour in the elevated-0-maze two days after exposure to the heat stress. Activity of the heat-exposed rats (as determined by using open field test) was also found to be decreased. Depression-like behaviour has previously been reported in the immature rat2829. Thus, our findings provide further evidence that hypocorticosteronaemia is associated with behavioural deficits in pre-pubertal male rats.
DHEA-S has been associated with adaptation against external stress31. Decrease in DHEA-S concentrations was reported in male subjects undergoing hot spring immersion (41°C) for 30 min14. In our study, adrenal androgen levels were below the limit of detection suggesting that only corticosterone secretion of the adrenal cortex was affected by 30-min heat stress in pre-pubertal rats.
It has been reported that exposure to hyperthermia during pregnancy caused marked growth retardation of the adrenal cortex and a decreased population of somatotropes in the adenohypophysis in the off-springs31. Immunoreactivity for ACTH in the pituitary gland of these animals was not significantly altered by hyperthermia. In our study, hyperthermia in 30- day old rats resulted in mild hydropic swelling and degeneration, respectively, in the adrenal cortex. Corticosterone secretion was significantly decreased in both groups 48 h after the heat stress exposure. It is possible that hyperthermia suppressed the function of the adrenal glands without remarkable change of their morphology. A recent study has shown that increased temperature decreases binding affinity of cortisol to plasma proteins32. Heat stress-related changes in glucocorticoid hormone levels may also be attributed to percentage of binding to the carriers rather than a change in secretion pattern.
In another study, rabbits were exposed to heat in a chamber similar to ours and the rectal temperature was monitored18 and acute hyperthermia resulted in reduced blood flow to the thyroid gland and decreased secretion of fT3 and fT4. In the present study, serum levels of thyroid hormones did not significantly differ compared to the control values as measured 48 h after heat exposure. Thus, it appears that hyperthermia causes a transient decrease in thyroid gland function. Immunoreactivity of the thyroid stimulating hormone in the pituitary gland of heat exposed foetuses was not significantly different from that of control specimens31. In our study, histopathology revealed hyperemia in the group I (39°C) and hydropic degeneration and focal necrosis in the group II (41°C). It appears that either these changes did not affect secretion pattern of pituitary-thyroid axis or any acute alteration in thyroid hormone secretion pattern was not sustained until 48 h after heat stress exposure.
Testicular function is highly dependent on temperature control and negatively influenced by hyperthermia20. Long-term application of mild testicular hyperthermia induces stage-specific and germ cell-specific apoptosis in adult monkey testes33. Similarly, exposure to heat for short period has been shown to trigger apoptosis in dividing cell populations in the testis21. In our study, induction of 41°C hyperthermia for 30 min in pre-pubertal rats has also caused pathological changes in sperm cells. Biochemical analysis revealed that serum TTE levels were below the limit of detection (<2.0 ng/dl) in all groups. Intratesticular testosterone plays a pivotal role in protecting germ cells against heat-induced cell death34. Thus, testes of pre-pubertal rats may be more susceptible to hyperthermia due to lack of protective effects of testosterone.
In conclusion, our findings suggest that heat stress may cause delayed reduction in serum corticosterone level which is associated with behavioural deficits in pre-pubertal male rats. Although thyroid hormones may be affected by hyperthermia, these seem to return to normal levels following a two-day recovery period.
References
- Modulation of physiological brain hyperthermia by environmental temperature and impaired blood outflow in rats. Physiol Behav. 2004;83:467-74.
- [Google Scholar]
- Methods to produce hyperthermia-induced brain dysfunction. Prog Brain Res. 2007;162:173-99.
- [Google Scholar]
- Human brain temperature: regulation, measurement and relationship with cerebral trauma: part 1. Br J Neurosurg. 2008;22:486-96.
- [Google Scholar]
- Hyperthermia induced pathophysiology of the central nervous system. Int J Hyperthermia. 2003;19:325-54.
- [Google Scholar]
- Heat shock transcription factors and the hsp 70 induction response in brain and kidney of the hyperthermic rat during postnatal development. J Neurochem. 2000;75:363-72.
- [Google Scholar]
- Cerebral neurons and glial cell types inducing heat shock protein Hsp70 following heat stress in the rat. Prog Brain Res. 2007;162:417-31.
- [Google Scholar]
- Effects of hypothermia and hyperthermia on attentional and spatial learning deficits following neonatal hypoxia-ischemic insult in rats. Behav Brain Res. 2004;151:209-17.
- [Google Scholar]
- Brief post-hypoxic- ischemic hypothermia markedly delays neonatal brain injury. Brain Dev. 1997;19:326-38.
- [Google Scholar]
- Influence of mild hypothermia on delayed mitochondrial dysfunction after transient intrauterine ischemia in the immature rat brain. Dev Brain Res. 2001;128:1-7.
- [Google Scholar]
- The effect of pre hypoxic-ischemic (HI) hypo and hyperthermia on brain damage in the immature rat. Dev Brain Res. 1999;117:139-43.
- [Google Scholar]
- Effects of hyperthermia on hypoxic-ischemic brain damage in the immature rat: its influence on caspase-3-like protease. Am J Obstet Gynecol. 2003;188:768-73.
- [Google Scholar]
- Alterations in cognitive performance during passive hyperthermia are task dependent. Int J Hyperthermia. 2011;27:1-9.
- [Google Scholar]
- Dehydroepiandrosterone sulfate linked to physiologic response against hot spring immersion. Steroids. 2009;74:945-9.
- [Google Scholar]
- Decreased heat tolerance is associated with hypothalamo-pituitary-adrenocortical axis impairment. Neuroscience. 2007;147:522-31.
- [Google Scholar]
- HPA and SAS responses to increasing core temperature during uncompensable exertional heat stress in trained and untrained males. Eur J Appl Physiol. 2010;108:987-97.
- [Google Scholar]
- The role of the hypothalamic-pituitary-adrenocortical system during inflammatory conditions. Crit Rev Neurobiol. 1994;8:263-91.
- [Google Scholar]
- Effect of hyperthermia on the function of thyroid gland. Eur J Appl Physiol. 2008;103:285-8.
- [Google Scholar]
- Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4 methylenedioxymethamphetamine (Ecstasy) J Pharmacol Exp Ther. 2003;305:159-66.
- [Google Scholar]
- Influence of genital heat stress on semen quality in humans. Andrologia. 2007;39:203-15.
- [Google Scholar]
- The effect of hyperthermia on the induction of cell death in brain, testis, and thymus of the adult and developing rat. Cell Stress Chaperones. 2002;7:73-90.
- [Google Scholar]
- HPA and sympathoadrenal activity of adult rats perinatally exposed to maternal mild calorie restriction. Behav Brain Res. 2010;208:202-8.
- [Google Scholar]
- Delayed melatonin administration promotes neuronal survival, neurogenesis and motor recovery, and attenuates hyperactivity and anxiety after mild focal cerebral ischemia in mice. J Pineal Res. 2008;45:142-8.
- [Google Scholar]
- Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141-9.
- [Google Scholar]
- Sex differences in salivary cortisol in response to acute stressors among healthy participants, in recreational or pathological gamblers, and in those with posttraumatic stress disorder. Horm Behav. 2010;57:35-45.
- [Google Scholar]
- Effect of hydration state on resistance exercise-induced endocrine markers of anabolism, catabolism, and metabolism. J Appl Physiol. 2008;105:816-24.
- [Google Scholar]
- Neuroendocrinology of post-traumatic stress disorder. Prog Brain Res. 2010;182:149-60.
- [Google Scholar]
- Is anxiety sensitivity a predictor of PTSD in children and adolescents? J Psychosom Res. 2008;65:81-6.
- [Google Scholar]
- Febrile convulsions in developing rats induce a hyperanxious phenotype later in life. Epilepsy Behav. 2006;9:401-6.
- [Google Scholar]
- Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114:187-93.
- [Google Scholar]
- Immunohistochemical study on the fetal rat pituitary in hyperthermia-induced exencephaly. Zoolog Sci. 2002;19:689-94.
- [Google Scholar]
- Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J Clin Endocrinol Metab. 2010;95:4689-95.
- [Google Scholar]
- Mild testicular hyperthermia induces profound transitional spermatogenic suppression through increased germ cell apoptosis in adult cynomolgus monkeys (Macaca fascicularis) J Androl. 2002;23:799-805.
- [Google Scholar]
- Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone on stage specificity. Endocrinology. 1999;140:1709-17.
- [Google Scholar]






