Translate this page into:
Assessment of effects on health due to consumption of bitter bottle gourd (Lagenaria siceraria) juice
Reprint requests: Dr G.S. Toteja, Scientist ‘F’ and Head (Nutrition), Indian Council of Medical Research, Ansari Nagar, New Delhi & Centre for Promotion of Nutrition Research and Training with special focus on North-East, Tribal & Inaccessible Population (ICMR), ICMR Campus II, 3 Red Cross Road, Tuberculosis Association of India Building, Ist floor, Near Parliament, New Delhi 110 001, India e-mail: gstoteja@gmail.com
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background & objectives:
The bottle gourd (Lagenaria siceraria) is popularly known as lauki, ghia or dudhi in India. Its consumption is advocated by traditional healers for controlling diabetes mellitus, hypertension, liver diseases, weight loss and other associated benefits. However, in last few years there have been reports of suspected toxicity due to consumption of its juice. This led to the constitution of an Expert Committee by Department of Health Research at Indian Council of Medical Research (ICMR), Ministry of Health & Family Welfare, Government of India in October 2010. The committee looked into the issues related to safety of consumption of bottle gourd juice, and this paper presents the findings.
Methods:
Information on cases of suspected toxicity due to consumption of bottle gourd juice was collected by internet search, advertising on website of ICMR and by writing to State and district health authorities as well as to medical colleges, hospitals and private nursing homes across the country.
Results:
Three deaths were reported, one from Delhi and two from Uttar Pradesh after consumption of extremely bitter bottle gourd juice. Three persons who died after consumption of freshly prepared bottle gourd juice or juice mixed with bitter gourd (karela) juice were over 59 years of age and had diabetes since last 20 years. This juice was reported to be extremely bitter by all three. Twenty six persons were admitted to various hospitals of the country on complaint of abdominal pain and vomiting following consumption of freshly prepared bottle gourd juice. Diarrhoea and vomiting of blood (haematemesis) was reported in 18 (69.2%) and 19 (73.1%) patients, respectively. Biochemical investigations revealed elevated levels of liver enzymes. More than 50 per cent patients had hypotension. Endoscopic findings showed profusely bleeding stomach with excessive ulceration seen in distal oesophagus, stomach and duodenum in most of the cases. All these patients recovered fully and no sequeale was recorded for any of the cases.
Interpretation & conclusions:
Cucurbitaceae family, of which bottle gourd is a member contains the toxic tetracyclic triterpenoid compounds called cucurbitacins which are responsible for the bitter taste. There is no known antidote for this toxicity and clinicians treat such cases symptomatically only. The Committee made the following recommendations: (i) The community needs to be educated that bitter tasting bottle gourd juice should not be consumed and in case there is any discomfort, nausea, vomiting, diarrhoea or any feeling of uneasiness after consumption of juice, the person should immediately be taken to a nearby hospital. (ii) Clinicians are suggested that patients coming with symptoms (discomfort, nausea, vomiting, diarrhoea, gastrointestinal bleeding after consumption of juice) should immediately be attended to and general supportive care should be provided, i.e. IV fluids/crystalloids/blood products/fresh frozen plasma to maintain the haemodynamics and electrolyte balance; Ryle's tube to be put in for gastric lavage and to assess gastrointestinal (GI) bleed- aspirate to be preserved; Proton pump inhibitors should be given for management of GI bleed and appropriate treatment for other complications should be given. (iii) The possible research areas identified are chemical composition studies on bitter and normal bottle gourd and other members of cucurbitaceae family; animal toxicity studies and studies on interaction between bottle gourd juice and other drugs.
Keywords
Bitter taste
bottle gourd
calabash
cucurbitacins
juice
Lagenaria sicraria
toxicity
The calabash or bottle gourd (Lagenaria siceraria (Molina) Standley) is popularly known as lauki, ghia or dudhi in India. In Ayurveda, bottle gourd is advocated for treatment of diabetes mellitus, hypertension, flatulence, cooling properties, liver diseases, weight loss and other associated benefits. It is part of complementary and alternative therapy which is widely prevalent in India12.
In June 2010, a 59 yr old male died in Delhi and his wife was hospitalized after they both drank a mix of bottle gourd (lauki) and bitter gourd (karela) juice. The matter was discussed in the Parliament, and Department of AYUSH, Ministry of Health and Family Welfare, gave an assurance in Lok Sabha stating that the entire matter will be investigated by a team of medical officers and scientists. To fulfill this, the Department of AYUSH requested Department of Health Research (DHR) to constitute an Expert Committee. DHR hence constituted an Expert Committee at Indian Council of Medical Research (ICMR), New Delhi, to look into the matter and give recommendations.
Methods
Archives of newspapers, news magazines, etc. available online were searched for any case reported allegedly due to consumption of bottle gourd juice. A call for information was also posted on the ICMR website seeking information from clinicians regarding cases reported, from general public regarding any incident, due to alleged consumption of lauki (bottle gourd) juice and from researchers on data generated, if any regarding the beneficial and toxic effects of bottle gourd juice. In addition, letters were written to the health authorities of all States and their districts as well as to the government and private medical colleges/ institutes, selected nursing homes, hospitals and clinicians requesting for any information on bottle gourd toxicity cases.
The committee was formulated in October 2010 and the report was submitted in August 2011.
Three deaths came to committee's knowledge due to alleged consumption of bottle gourd (lauki) juice, one in Nanakpura, Delhi on June 23, 2010 and two in Amroha (District J.P. Nagar), Uttar Pradesh on March 28, 2007. A team of experts consisting of a pharmacologist and gastroenterologist from AIIMS, New Delhi, Medicine expert from Hamdard Institute of Medical Sciences and Research, New Delhi and nutrition expert from ICMR, New Delhi visited Nanakpura. Whereas the family in Amroha was visited by a team of pharmacologist from AIIMS, New Delhi, medicine expect from Hamdard Institute of Medical Science and Research, New Delhi, nutrition expert from ICMR, New Delhi, District Health Authority and other representatives. The hospitals where these patients were taken were also contacted.
All information including published reports/articles was reviewed and debated by Task Force members and other experts for making appropriate recommendations.
Results & Discussion
Extensive efforts were made to collect information from various parts of India on suspected cases of toxicity due to alleged consumption of bottle gourd juice. The Governments of 17 States and Union Territories, 81 district health authorities and 110 government and private medical colleges, hospitals and nursing homes informed that no case of bottle gourd toxicity has been reported in their s0 tates/districts/medical colleges/hospitals.
Three deaths were reported and information was received on 26 cases who got admitted to the hospitals and recovered. Except one case, all others were reported from private sector hospitals/nursing homes.
The 26 cases who were admitted in different hospitals of the country after consumption of bitter juice were from National Capital Region (16), Madhya Pradesh (7), Gujarat (2) and Uttarakhand (1).
Death due to alleged consumption of bottle gourd juice: One case of death (59 yr, male) was reported from Nanak Pura, a Government Colony in South Delhi on June 23, 2010. This person had diabetes for the last 30 years and he has been consuming a mix of bottle gourd and bitter gourd juice (150-175 ml) for two and a half years before his death. On June 23, 2010, he consumed the juice at 0730 h after taking a cup of tea with a couple of rusks. The juice was extremely bitter as compared to other days. Within five minutes of consumption of juice, he became uncomfortable, giddy and started vomiting. He also had diarrhoea and passed blood in stools. He was ultimately taken to a private hospital in South Delhi at around 1130 h where he was declared brought dead by the hospital authority. Except diabetes, he had no other major health problem.
According to the postmortem report, the stomach and small intestine were found to be intensely haemorrhagic, reddish in colour with velvety feel, mucous mixed, blood present. Further, the large intestine was also intensely haemorrhagic and was reddish in colour. The spleen, liver and both the kidneys were found to be pale. No abnormality was observed in the heart and the chambers contained dark colour blood. The viscera were preserved and sent to a forensic laboratory of Government of Delhi for common unknown and arsenic poisoning.
Two other deaths were reported from Amroha, District J.P. Nagar (UP) where a couple (husband and wife) died on on March 28, 2007 after consuming bottle gourd juice. The husband 68 yr had diabetes for the 20 yr and started consuming juice only eight days before his death (100 ml/day). On the day of the incident, he consumed approximately 20 ml freshly prepared juice of bottle ground at around 0830 h, against 100 ml consumed normally as the juice was extremely bitter. Five minutes after consuming the juice, he started vomiting and also had diarrhoea. There was blood in his stool and vomiting. He was taken to a private hospital in Moradabad (U.P).
His wife (59 yr old) also a diabetic for the last 20 years, consumed 100 ml of bottle gourd juice on March 28, 2007. She also reported the juice to be extremely bitter. After five minutes of consuming the juice, she also developed same symptoms as her husband as was admitted to the same private hospital in Moradabad.
According to the hospital sources they were brought to the hospital around 1115 h both were known cases of diabetes and hypertension. Both were found having septicaemia and renal failure on investigation. Patients were referred to higher centre on request by family but died before being shifted (Table I).
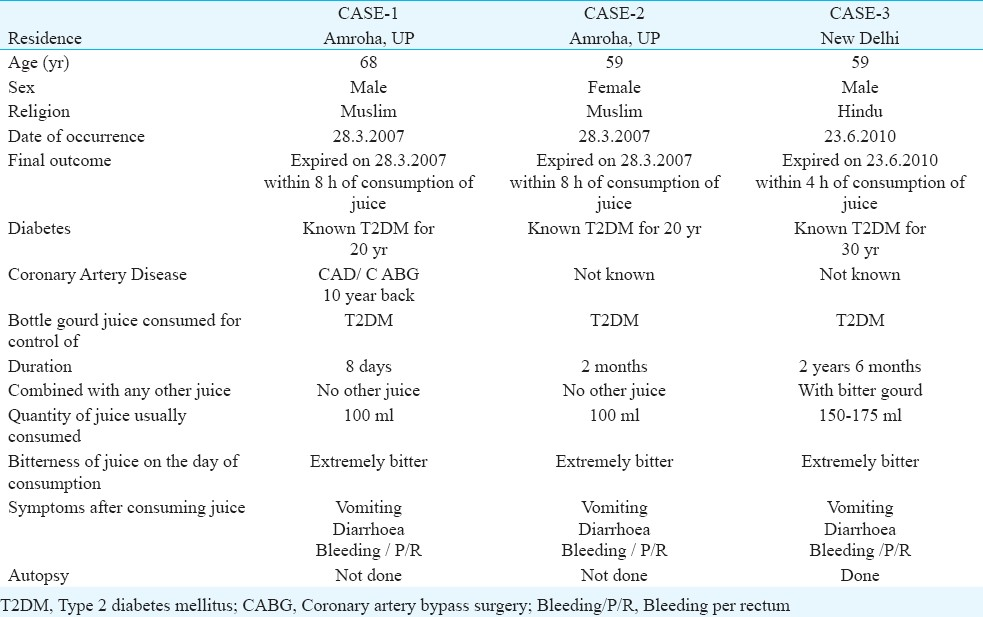
Cases admitted to hospital after consumption of bottle gourd juice: Information could be collected for 26 cases (17 males, 9 females) of suspected toxicity of consumption of bottle gourd juice from all over the country (age range 30-73 yr). The duration of consumption of juice varied from a couple of week to over 20 years.
On the day of the incident, the patients (n=26) consumed between 20-350 ml of freshly extracted bottle gourd juice which was reported to be bitter. One patient had boiled the juice before consumption. Only a couple of patients reported to be consuming the juice on an empty stomach, whereas for others this detail was not available. In three cases, the juice was mixed with juices of either beetroot or bitter gourd and the remaining consumed only bottle gourd juice. One important finding was that in all cases the bottle gourd juice was reported to be unusually bitter, however, the appearance (type, shape and colour) of bottle gourd used was similar to that used on other days.
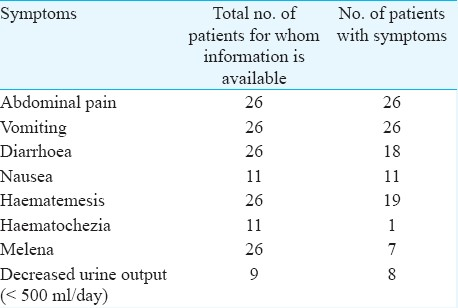
Symptoms - All patients reported severe abdominal pain accompanied by vomiting with or without blood after consumption of juice and 18 of them reported diarrhoea. The time of development of symptoms varied from five min to as long as 9 h. Melena and haematochezia was observed in 26.9 per cent (7 of 26) and 9.1 per cent (1 of 11) cases, respectively. Renal function was also affected with a decreased urine output in 88.9 per cent (8 of 9) cases (Table II).
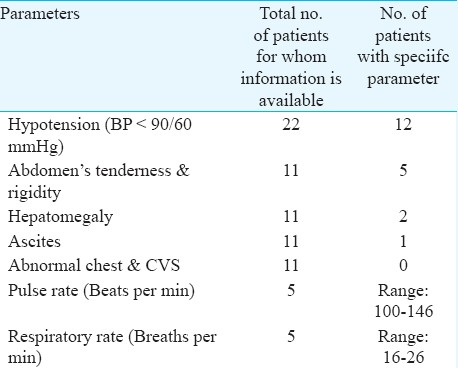
Clinical examination - In general, the patients were conscious and well oriented with only a few being drowsy and disoriented. Five patients had tachycardia. Of the 22 patients for whom record of blood pressure was available, 12 (54.5%) were found to be hypotensive at the time of reporting to the hospital. In most of the cases, no hepatosplenomegaly and ascites were revealed on examination (Table III).
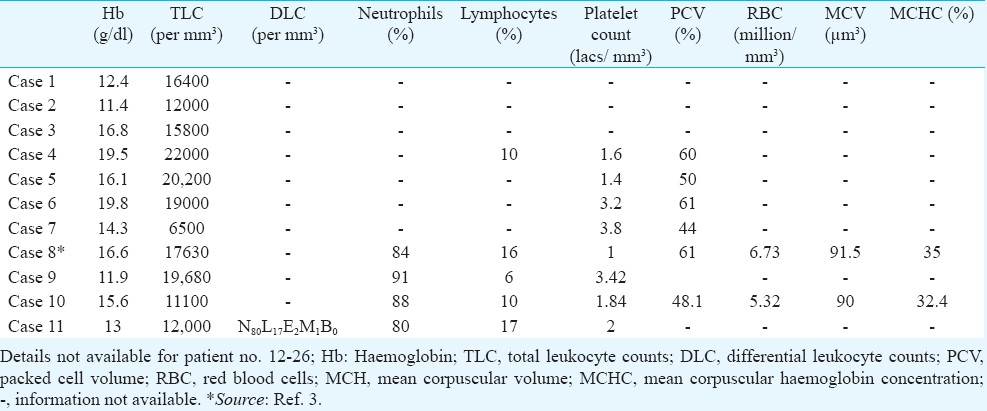
Investigations: Biochemical investigations: Limited data on the biochemical investigations were available for only 11 patients from the hospital records. Increased haemoglobin and packed cell volume (PCV) were observed in a few patients being indicative of haemo-concentration resulting from either internal or external fluid loss. Elevated TLC was also observed in most of the patients (Table IV). Serum electrolyte (sodium and potassium) levels were normal in all patients except two cases where either sodium or potassium levels were high (Table V). Nine out of the ten patients had elevated liver enzymes (SGPT and SGOT) attributable to either cytotoxic effect or ischaemia to liver. Bilirubin levels were normal in all patients (Table VI).
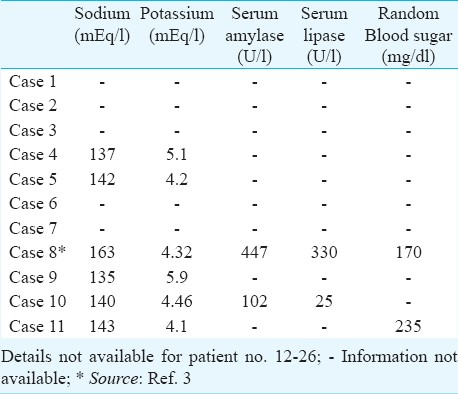
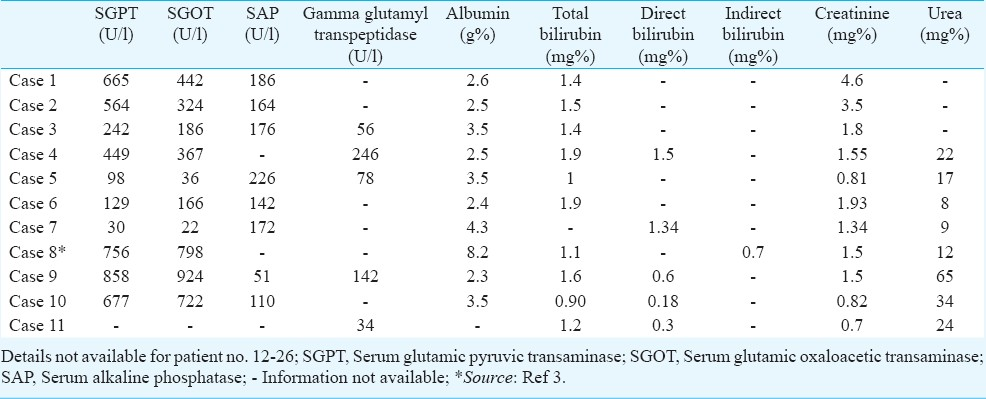
Others - Endoscopic findings revealed profusely bleeding stomach with excessive ulceration in distal oesophagus, stomach and duodenum in most of the cases. In one case the vocal cords and epiglottic folds were also found to be ulcerated. Most of the lesions were seen in the body and antrum of the stomach.
Treatment and outcome - All cases of bottle gourd toxicity required hospitalization with the duration of stay ranging from 1 to 12 days. A total of 18 patients had to be initially managed in an intensive care unit for 1 to 4 days. All the patients recovered fully with no sequeale. The renal and liver functions returned to normal in all patients and mucosal changes on endoscopy reverted to normal within 7 days.
The natural cucurbitacins constitute a group of diverse triterpenoid substances which are known for their bitterness and toxicity. Structurally, these are characterized by the tetracyclic cucurbitane nucleus skeleton, namely, 19-(10→9β)-abeo-10-α-lanost-5-ene (also known as 9β-methyl-19-nor lanosta-5-ene), with a variety of oxygenation functionalities at different positions. According to the characteristics of their structures, cucurbitacins are divided into twelve categories4. The bottle gourd fruit contains the triterpenoid cucurbitacins B, D, G, H and 22-deoxy cucurbitacin5. Higher levels of cucurbitacin are triggered by environmental stress, like high temperatures, wide temperature swings or too little water, uneven watering practices, low soil fertility and low soil pH5–7.
David and Vallance reported intraperitoneal median lethal dose (LD50) for pure cucurbitacin (Cuc) isolated from species of Cucumis in albino mice as 1.2 mg Cuc A/kg; 1.0 mg Cuc B/kg and 0.8 mg Cuc C/kg and in the rat 2.0 mg Cuc A/kg with lethal amounts causing acute pulmonary oedema8. Apart from bitter bottle gourd, several cases have been reported due to consumption of other vegetables of Cucurbitaceae family. Twenty two cases of food poisoning associated with intensely bitter zucchini were recorded by Queensland health authorities (Australia) between November, 1981 and December, 1982. Symptoms of illness were apparent 1 to 2 h after consumption. Ingestion of about 3 g of bitter zucchini was sufficient to incite nausea followed by collapse, with severe stomach cramps lasting 3 days, and diarrhoea which persisted longer. Vomiting was seldom associated9. In 2003, in Dodge county, Nebraska, a gardener had severe stomach cramps and diarrhoea for several days following ingestion of extremely bitter zucchini squash7.
The Committee noted that the common finding was that the juice consumed on the day of the incident was unusually bitter. Thus, it seems that none of the patients had the prior knowledge that a bitter tasting juice is to be discarded. All patients after consumption of the juice had vomited and reported abdominal pain.
After going through the available details, the Expert Committee recommended that the following advisory should be issued:
For public: The community needs to be educated regarding the following: (i) A small piece of bottle gourd should be tasted before extracting the juice to ensure that it is not bitter. If bitter, it should be discarded. (ii) Bitter bottle gourd juice should not be consumed at all. (iii) Bottle gourd juice should not to be mixed with any other juice. (iv) In case of discomfort after consumption (nausea, vomiting, diarrhoea or any feeling of uneasiness), the person should be immediately taken to a nearby hospital.
For clinicians: Any case which comes with symptoms of any discomfort, nausea, vomiting, diarrhoea, gastrointestinal (GI) bleeding after consumption of bottle gourd juice should immediately be attended to and the following assessment should be carried out after securing an IV route:
-
Detailed clinical examination with recording of vitals. Details of the quantity of juice consumed and its taste should also be recorded.
-
Haemogram, urine examination, biochemical: electrolytes, liver and kidney function tests including prothrombin time and platelet counts, serum amylase, blood sugar.
-
Others: X-Ray chest, ECG, ultrasound and endoscopy as and when required.
Principles of management: There is no specific antidote available, therefore, the following measures are suggested:
-
General supportive care: IV fluids/crystalloids/blood products/fresh frozen plasma to maintain the haemodynamics and electrolyte balance.
-
Ryle's tube to be put in for gastric lavage and to assess GI bleed- aspirate to be preserved.
-
Proton pump inhibitors for management of GI bleed.
-
Appropriate treatment for other complications.
Scientific studies are available in the literature highlighting the beneficial properties of bottle gourd (L. siceraria) on human health510–15. However, limited information is available on the adverse effects, if any on consumption of its juice. The possible research areas identified are (i) to conduct chemical composition studies on bitter and normal bottle gourd and other members of Cucurbitacae family, (ii) animal toxicity studies, and (iii) studies on interaction between bottle gourd juice and other drugs.
References
- Phytochemical and pharmacological review of Lagenaria siceraria. J Ayurveda Integr Med. 2010;1:266-72.
- [Google Scholar]
- Cucurbitacins and cucurbitane glycosides: structures and biological activities. Nat Prod Rep. 2005;22:386-99.
- [Google Scholar]
- Phytopharmacological profile of Lagenaria siceraria: A review. Asian J Plant Sci. 2010;9:152-7.
- [Google Scholar]
- Bitterness in Zucchini Squash and Cucumber. Available from: http://cuke.hort.ncsu.edu/cucurbit/cuke/cukehndbk/cukebitterness.html
- [Google Scholar]
- Redlands Horticultural Research Station, Ormiston, Qld. 4163, Australia, appeared in “Cucurbit Genetics Cooperative Report No. 6 (1983) Available from: file:///C:/Documents%20and%20Settings/ICMR/Desktop/FOOD%20SAFETY/LAUKI%20JUICE/MEETINGS/1st%20MEETING%2021.12.2010/LITERATURE/Herrings.htm
- [Google Scholar]
- Lagenaria siceraria: Phytochemistry, pharmacognosy and pharmacological studies. Rep Opin. 2010;2:91-8.
- [Google Scholar]
- Antihyperlipidemic effect of the methanolic extract from Lagenaria siceraria Stand. fruit in hyperlipidemic rats. J Ethnopharmacol. 2009;124:333-7.
- [Google Scholar]
- Central nervous system activity of different extracts of Lagenaria siceraria (Mol) Standl leaves parts. Int J Pharma Research Development. Available from: http://www.ijprd.com/CENTRAL%20NERVOUS%20STSTEM%20ACTIVITY%20OF%20DIFFERENT%20EXTRACTS%20OF%20LAGENARIA%20SICERARIA%20(MOL)STANDL.%20PARTS.pdf
- [Google Scholar]
- Analgesic and anti-inflammatory activities of Lagenaria siceraria Stand. fruit juice extract in rats and mice. Pharmacognosy Mag. 2006;2:232-8.
- [Google Scholar]
- Free radical scavenging activity of Lagenaria siceraria (Mol.) Standl. fruit. Nat Prod Radiance. 2007;6:127-30.
- [Google Scholar]
- Beneficial effects of Lagenaria siceraria (Mol.) Standley fruit epicarp in animal models. Indian J Exp Biol. 2008;46:234-42.
- [Google Scholar]






