Translate this page into:
Assessment of diagnostic accuracy of Gazelle: A point-of-care testing device for screening β-thalassemia trait
For correspondence: Dr Tulika Seth, Department of Haematology, All India Institute of Medical Science, New Delhi 110 029, India e-mail: drtulikaseth@gmail.com
-
Received: ,
Accepted: ,
Abstract
Background & objectives
Timely detection of population with β-thalassemia trait (BTT) followed by genetic counselling is an advocated method of preventing the birth of a child with β-thalassemia major. In this study we aim to assess the diagnostic accuracy of Gazelle, a point-of-care (POC) testing device, in screening for BTT in hospital laboratory setting.
Methods
Standards for Reporting Diagnostic Accuracy (STARD) guidelines were followed in developing study design, recruiting study participants and sample size calculation for the current research. A consecutive sample of 446 participants was recruited for this study and was tested for the reference test Gazelle as well as the gold standard cation exchange high performance liquid chromatography (CE-HPLC). Low serum ferritin levels are known to interfere with the production of HbA2 and in turn lead to false negative results. Hence, the study population was divided into two categories with respect to a cut off value of 15ng/dl of serum ferritin and the results were analyzed.
Results
Overall diagnostic accuracy of Gazelle for detecting BTT was found to be 95.3 per cent with a confidence interval (CI) of (92.9 - 97.1) and the sensitivity was 94.5 per cent with a 95% CI of (84.8 - 98.8). When analyzed by the serum ferritin level the diagnostic accuracy was found to be 94.7 per cent (91.1% - 97.1%) and 95.7 per cent (91.8% - 98.1%) for participants with serum ferritin level as > 15 ng/ml and < 15 ng/ml, respectively.
Interpretation & conclusions
This study found Gazelle to be a good screening tool for β-thalassemia trait with high sensitivity, specificity and accuracy. However, it is recommended that the final confirmation of the diagnosis done by a diagnostic test like HPLC or Capillary Zone Electrophoresis (CZE).
Keywords
Point-of care testing
device validation
β-thalassemia trait
screening
diagnostic accuracy
Hb A2
β-thalassemia is a haemoglobin disorder characterized by defects in the haemoglobin β-globin chain and has autosomal recessive pattern of genetic inheritance1. β-thalassemia major patients have a mutation in both the genes responsible for β-globin chain and thus experience severe symptoms and require medical care throughout their life including routine blood transfusions and iron chelation therapies1. In contrast, β-thalassemia trait (BTT) only has one affected gene and the other gene responsible for coding the β-globin chain of haemoglobin functions normally. Hence, individuals with BTT have mild or no anaemia along with variable microcytosis2.
Approximately 1.5 per cent of the global population is affected by β-thalassemia and approximately 40,000 newborns are added to this pool every year3,4. Populations from regions of Africa, South East Asia, Middle East and southern European are known to have >90 per cent of the world’s burden of β-thalassemia5. Timely detection of population with BTT followed by genetic counselling is the most advocated method of preventing the birth of a child with β-thalassemia major. However, large-sale population screening of β-thalassemia has not been implemented due to logistically intensive and costly nature of the diagnostic tests6.
Currently the technology available for screening of β- thalassemia is a Naked Eye Single Tube Red Cell Osmotic Fragility Test (NESTROFT) which suffers from several shortcomings like requirement of freshly prepared buffer each time to false negative results in cases of severe anaemia7. The other option is using cut off values for mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) parameters generated from automated haematology analyzers which can be done only in health care facility setting. The laboratory diagnostic tests for β-thalassemia are high performance liquid chromatography (HPLC), haemoglobin electrophoresis or capillary zone electrophoresis, which require specialized equipment along with highly trained health care personnel for conducting as well as interpretating the test result. Therefore, screening and diagnosis of β-thalassemia pose a challenge in low resource setting, where it is most prevalent3. A portable device, which gives valid results in real time, can be game changer as it can enhance the coverage for population screening of β- thalassemia.
Gazelle (Hemex health, Portland, USA) is a miniature haemoglobin electrophoresis device which has been validated for the detection and quantification of sickle variant of haemoglobin in various settings8-10. The device has now incorporated an image analysis algorithm for detection as well as quantification of haemoglobin A2 (HbA2). This study was undertaken to assess the diagnostic accuracy of Gazelle in screening for BTT in hospital laboratory setting. To the best of our knowledge this is the largest study undertaken for the validation of a POCT device for thalassemia trait.
Material & Methods
Study design
A prospective diagnostic accuracy validation study was undertaken in hospital laboratory setting by the department of Haematology, All India Institute of Medical Sciences (AIIMS), New Delhi. The investigational assay was Gazelle (Hemex health, Portland, USA), and the reference (gold-standard) test was cation exchange high performance liquid chromatography (CE-HPLC) using the VARIANTTM II β-thalassemia Short Program (Bio-Rad, Hercules, CA). The study was designed, implemented, and supervised at AIIMS, New Delhi, India. The ethical approval was obtained from the Institutional Review Board before conducting the study. The Gazelle reader and cartridge were developed by Hemex health, USA and were provided without charge for this study.
Study population
All individuals >1 yr of age, registered at paediatric outpatient department, antenatal clinic and in-patient department of the tertiary care hospital who were advised to undergo HPLC test by their treating doctor were enrolled in this study after taking an informed consent. Anyone with a history of blood transfusion in the last three months was excluded from the study. A total of 453 participants were approached for the study, out of which 446 gave consent and participated in the study. A consecutive sample of 446 participants was recruited for the study and was tested for the reference test Gazelle as well as the gold standard CE-HPLC.
Study methods
Standards for Reporting Diagnostic Accuracy (STARD) guidelines were followed in developing study design, recruiting study participants and sample size calculation for the current research11. A sample of consecutive participants reporting at study site between January to June 2023 were included in the study after obtaining informed consent. Estimation of sample size for this study was in accordance with published methodology by incorporating expected positivity rate for β-thalassemia among the tested samples at hospital laboratory to be 10 per cent (unpublished data) into sample size calculation with 95 per cent confidence interval and estimates taken for sensitivity and specificity of the candidate devices12. Sensitivity of paper based microchip electrophoresis device (Gazelle) for screening of β-thalassemia was reported between 97-100 per cent in an internal study conducted by Hemex13. For the purpose of calculation the lower limit of confidence interval for sensitivity (97%) was considered which resulted in a minimum sample size of 446.
Specifications and conduct of tests
2 ml of peripheral blood sample was collected in a vacutainer containing EDTA as an anticoagulant from all the participants of the study. All samples were tested for complete blood count, serum ferritin estimation, Hb fraction estimation by Gazelle and CE-HPLC.
Gazelle
A detailed description of development and design of Gazelle (hemechip) device has been published previously8. In brief, Gazelle is a miniaturized version of the haemoglobin electrophoresis test method with a single-use disposable test cartridge incorporating a cellulose acetate test strip.The different hemoglobin fractions migrate and separate into visible bands on the cellulose acetate paper which enables identification (normal, trait, disease) as well as quantification of hemoglobin as Hb A (normal), Hb S (sickle), Hb F (foetal) and Hb (A2/C/E). In about eight min, the interpretation as well as the types and percentages of haemoglobin are displayed on the screen and the results are also stored in the reader’s memory. The reports once generated can be printed or shared through wireless data transfer medium using WiFi or Bluetooth. The device operates on lithium batteries as well as electric power supply. Once charged fully, the batteries can provide back up for 7–8 h. The Gazelle test was conducted by a laboratory staff holding a diploma in the field and a work experience of two yr.
CE-HPLC
In principle CE-HPLC identifies haemoglobin variants using their retention time in their respective window after separating them by cation exchange chromatography. The test was conducted as per the manufacturer’s instructions for the Bio Rad VARIANT II β-thalassemia Short Program by an experienced laboratory technician qualified with a graduation in laboratory technology. CE-HPLC graphs were interpreted by Hematopathologist at the institute and the results entered in google sheet.
Blinding and compilation of results
All positive as well as 10 per cent of negative results were randomly cross checked by our expert (TS) at AIIMS, New Delhi. The reference test for Gazelle was conducted at the sample collection site only while; CE-HPLC, CBC and Serum Ferritin test were conducted in batches as done routinely at the laboratory. Reference test results were not available to the HPLC technicians or to the experts at the time of interpretation of CE-HPLC graphs. Furthermore, the results of HPLC test were blinded from the technician performing tests on Gazelle. After completion of data collection, results of the tests conducted on Gazelle were retrieved from device memory and entered in the data sheet for analysis. In case of any discordance the CE-HPLC results were taken as final.
Data analysis
There were 28 test results which read as inconclusive and a prompt of retest was displayed on Gazelle reader. The reason for inconclusive result was inadequate lysis of the sample or insufficient processing and all the samples were tested again and results included in the study. Bland-Altman analysis was done to assess the agreement of quantification of HbA2 by Gazelle in comparison to HPLC.
Results
A total of 446 blood samples were tested in the current study and 112 (25%) of which belonged to males and rest were females. The distribution of study participants as per gender, age group and reason for advising HPLC test are shown in Table I. The age range for the study participants was from 2-68 yr with maximum participants in the range of 18-50 yr. The haemoglobin range of the participants was 4.1 to 15.1 g/dL with a mean of 10.7 (+3.5) g/dL. The serum ferritin level of participants was 10–2800 ng/ml.
| n (%) | |
|---|---|
| Gender | |
| Male | 112 (25.1) |
| Female (non-pregnant) | 141 (31.6) |
| Female (pregnant) | 193 (43.2) |
| Age (yr) | |
| 2-18 | 103 (23) |
| >18-50 | 320 (71.7) |
| >50 | 23 (5.1) |
| Haemoglobin (mean ± SD) | 10.7 (+3.5) |
| Reason for advising HPLC | |
| Antenatal screening | 193 (43.2) |
| Family screening | 159 (35.6) |
| Investigation for cause of anaemia | 94 (21) |
| Total | 446 |
SD, standard deviation; HPLC, high-performance liquid chromatography
All the 446 blood samples were tested by Gazelle as well as HPLC along with estimation of CBC and serum ferritin. Table II presents the cross tabulation of results of estimation of HbA2 by Gazelle and CE-HPLC. Since low serum ferritin levels are known to interfere with the production of HbA2 and in turn lead to false negative results, the study population was divided into two categories with respect to a cut off value of 15ng/dl of serum ferritin and the results were analyzed. The HbA2 range for diagnosing confirmed cases of BTT was in the range of >4-9 per cent of haemoglobin fraction, while those between 3.5-4 per cent were considered as borderline with a possibility of BTT. We took a cut off range of 3.6-9 per cent for validation of Gazelle as a screening test for BTT so as to include all likely cases of BTT. Total 52 sample results from Gazelle were found concordant with results from HPLC and only three cases in the HbA2 range of 3.5-9 per cent detected by CE-HPLC were missed by Gazelle. In total, 70 samples were detected in the range of BTT by Gazelle, while CE-HPLC detected 55 cases with HbA2 in the specified range among the study participants. Among the 15 samples misdiagnosed by Gazelle as cases of BTT nine had serum ferritin levels >15 ng/dl while six had serum ferritin levels <15 ng/dl. Among them is diagnosed samples seven were being evaluated for cause of anaemia, five were part of family cascade screening and three were ante natal cases.
| Candidate test | Hb A2 value | CE-HPLC HbA2 | Total | ||
|---|---|---|---|---|---|
| <3.5% | 3.6%-9% | >9.1% | |||
| Comparison of HbA2 results of all tested | |||||
| Gazelle HbA2 | <3.5% | 360 | 2 | 0 | 362 |
| 3.6-9% | 13 | 52 | 5 | 70 | |
| >9.1% | 1 | 1 | 12 | 14 | |
| Total | 374 | 55 | 17 | 446 | |
| Comparison of HbA2 results of participants with serum ferritin >15 ng/ml | |||||
| Gazelle HbA2 | <3.5% | 183 | 1 | 0 | 184 |
| 3.6-9% | 7 | 42 | 4 | 53 | |
| >9.1% | 1 | 1 | 10 | 12 | |
| Total | 191 | 44 | 14 | 249 | |
| Comparison of HbA2 results of participants with serum ferritin < 15 ng/ml | |||||
| Gazelle HbA2 | <3.5% | 177 | 1 | 0 | 177 |
| 3.6-9% | 6 | 10 | 1 | 17 | |
| >9.1% | 0 | 0 | 2 | 2 | |
| Total | 183 | 11 | 3 | 197 | |
Bland-Altman analysis14 was done for all the samples detecting HbA2 (Figure). This method was used to assess the agreement of quantification of HbA2 by Gazelle in comparison to HPLC 1.96 times the standard deviations (SD) of the differences between the measurements of Gazelle and HPLC was set as the coefficient of repeatability. The mean difference among Hb A2 values detected by Gazelle and HPLC was found as 0.359 with an SD of 3.885. The dispersion of variables was studied using a scatter plot. The upper limit of agreement was calculated using mean + 1.96x SD (7.973) and the lower limit of agreement was calculated using mean – 1.96x SD (–7.2556). The Bland-Altman plot showed the mean bias ± SD between HbA2 levels as measured by Gazelle and HPLC first as 0.359±3.885, and the limits of agreement were −7.2556 and 7.973.
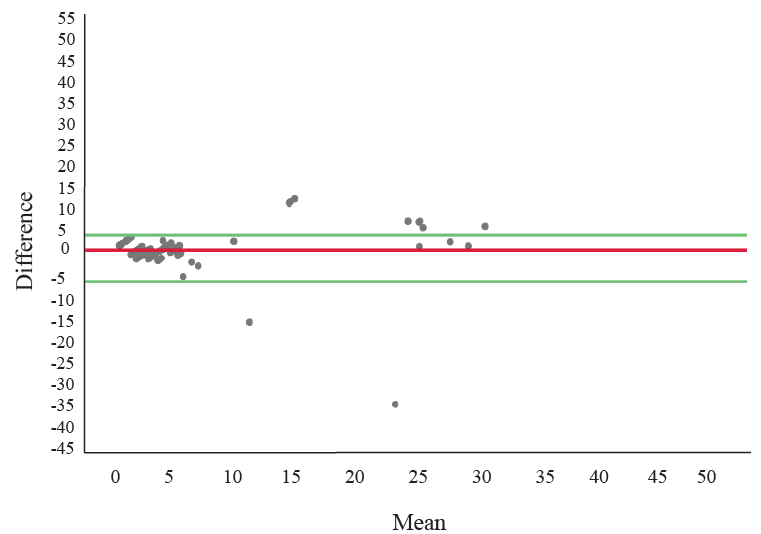
- Bland Altman plot assessing the agreement in quantification of HbA2 between Gazelle and HPLC. Note: X axis is mean of Hb A2 estimation by HPLC and Gazelle and Y axis is difference of HbA2 estimation between the tests.
Diagnostic accuracy of Gazelle in comparison to HPLC for estimating Hb A2 was determined for the group as well as the subgroups based on serum ferritin (Table III). The overall accuracy remained comparable, while sensitivity had dropped in the group with reduced serum ferritin levels. The negative predictive value of the test remained high (>98%), thus it was observed that it fits into the role of a screening test.
| 95% CI | |||||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | |
| All tested | 94.5 (84.8 - 98.8) | 95.4 (92.8 - 97.2) | 69.5 (59.1 - 78.2) | 99.3 (98.1 - 99.7) | 95.3 (92.9 - 97.1) |
| Serum ferritin > 15 ng/ml | 95.4 (84.5 - 99.4) | 94.6 (90.6 - 97.2) | 66.4 (52.5 - 77.9) | 99.4 (97.9 - 99.8) | 94.7 (91.1 - 97.1) |
| Serum ferritin < 15 ng/ml | 90.9 (58.7 - 99.7) | 96.2 (92.3 - 98.4) | 72.8 (55.9 - 85.4) | 98.9 (93.6 - 99.8) | 95.7 (91.8 - 98.1) |
CI, confidence interval
The sample with HbD-Punjab trait was interpreted as HbS trait, which is an inherent lacuna of the electrophoresis technology. Although, Gazelle was able to flag almost all the samples with haemoglobinopathies, the final diagnosis confirmation would require testing by some diagnostic tests HPLC, IEF or capillary zone electrophoresis.
Discussion
In the current study we assessed the diagnostic accuracy of Gazelle for HbA2 quantification as compared to CE-HPLC taken as gold standard. We found sensitivity, specificity and diagnostic accuracy of 94.5 per cent, 95.4 per cent and 95.3 per cent, respectively. The currently available modalities for screening of BTT includes NESTROFT, identification of microcytosis and hypochromia based on cut off values for MCV and MCH along with formula based indices derived from erythrocytic parameters. The diagnosis of BTT is typically confirmed by CE- HPLC and capillary electrophoresis.2
NESTROFT exposes the erythrocyte to saline solution and assesses their resistance to hemolysis7. The degree of haemolysis is interpreted as positive by presence of hazy or cloudy appearance of solution on visual inspection after 15 to 30 min of adding blood sample to the saline solution. This is a simple, fast, and inexpensive method used for preliminary screening of thalassemia carrier status and provides good sensitivity and moderate specificity15-19. Moreover, the test result is known to be affected by compound heterozygous inheritance of other haemoglobin variants, alpha thalassemia, glucose-6-phosphate dehydrogenase deficiency leading to false-positive results19,20. Gazelle conducts tests on the principle of electrophoresis and hence has the advantage NESTROFT that it can identify as well as quantify the haemoglobin fractions present. Also, unlike NESTOFT the results of HbA2 testing by Gazelle are not affected by the iron deficient state. The quality of tests conducted by NESTROFT is dependent on the quality of the buffer whereas in Gazelle most of the consumables are prepacked and available with the kit hence no such variability is encountered. However, correct diagnosis in Gazelle is compromised in presence of HbE which co-elute in the same window on electrophoresis. NESTROFT is a simple test to conduct and does not involve much training, whereas some training is required for conducting tests on Gazelle. Furthermore, Gazelle either requires an electric power source for its functioning or the use of rechargeable battery provided with it which gives a backup of 7-8 h.
Gazelle has been validated for screening of sickle cell trait in various settings and hence is capable of detecting multiple types of haemoglobinopathy with a single test, unlike the tests for detecting the presence of β-thalassemia trait or haemoglobin variant S or haemoglobin E which need to be done separately8-10. In an earlier study using Gazelle in India, a high specificity and accuracy was demonstrated to identify homozygous sickle cell disease, β-thalassemia major and sickle cell trait. However, this study did not include any patients of BTT and compound heterozygous β-thalassemia.
The impact of iron deficiency on lowering of HbA2 levels can be a challenge in diagnosis of β-thalassemia trait. We divided our samples in two groups based on ferritin levels to identify the impact of iron deficiency on Hb A2 estimation by Gazelle. The diagnostic accuracy was found to be comparable between the two groups irrespective of serum ferritin levels. Similar findings have been reported previously where HbA2 levels of iron replete individuals was comparable to those with iron deficiency21-23.
There is a debate concerning rise of HbA2 levels during pregnancy among women not having BTT. A study comparing the HbA2 levels among pregnant and non-pregnant women not having BTT reported that there is a slight increase in the level of HbA2 among pregnant women but not to a level higher than 3.5 per cent thus not interfering with the diagnosis of BTT24.
Erythrocyte parameters like MCV and MCH have been used with varying cut offs to screen BTT, however, similar blood count findings of iron deficiency anaemia pose a challenge in diagnosis unless used with a simultaneous estimation of serum ferritin25-25. The estimation of MCV and MCH are provided from an automated blood cell analyser, which is rapid and easy to interpret. However, multiple conditions like anaemia due to iron deficiency and inflammation can affect RBC indices, especially MCV, and these values could be as low as those found for thalassemia traits25. In addition, the erythrocytes are known to swell up on storage and hence MCV may give false negative results on stored samples26. The automated haematology analyzer used for the indices is facility based and does not have portability, also there is requirement of a trained laboratory technician to conduct the test. Gazelle testing device on the other hand is portable and the testing can be conducted after a training of 1-2 days given to any health care worker. Furthermore, the limitation of overlap with the diagnosis of iron deficiency is not there with the use of Gazelle.
A study27 conducted at a laboratory in Mumbai, India, under the aegis of Hemex health to determine the diagnostic accuracy of Gazelle for detecting BTT with HPLC taken as gold standard tested 400 samples of individuals between 4–64 yr age. Matched samples for thalassemia intermedia and trait were included. They reported a sensitivity of 100 per cent and specificity of 98.6 per cent for detection of BTT by Gazelle27.
On the other hand study28 done in Chhattisgarh and Madhya Pradesh, India for evaluating the diagnostic accuracy of Gazelle for detecting sickle haemoglobin with the reference test taken as standard cellulose acetate electrophoresis and all positive cases again subjected to HPLC. This study reported all the detected haemoglobin variants among the study participants and stated that β-thalassemia major individuals were misidentified as Hb SS disease by Gazelle. Furthermore, five samples with haemoglobin variant as sickle traits (Hb AS) were misidentified as normal (Hb AA) due to light S bands, or SS and SF. Three individuals with no abnormal Hb (Hb AA) were reported as AS and as SS by Gazelle. However, overall Gazelle yielded a high diagnostic accuracy (99%) for detecting sickle haemoglobin as compared to reference standard tests (HPLC and haemoglobin electrophoresis). The results of this study also conveyed the requirement of a follow up with a confirmatory test like HPLC after a positive diagnosis by Gazelle28.
The confirmatory tests for β-thalassemia trait are HPLC and capillary zone electrophoresis, both require expensive equipment and well trained professionals for conducting and interpretating the test results. The setup for these tests involves heavy equipment which can only be based in laboratory settings with specific conditions for power supply and temperature control. On the other hand, the set up for Gazelle consists of a portable reader which can be used with constant electricity supply or with a battery backup of 7-8 h when fully charged. Gazelle is a simpler to operate apparatus and gives printable report within minutes of testing. The device can also be connected with internet for sharing reports through email. The turnaround time for each test is about 10-12 min.
To the best of our knowledge the current study is the largest study undertaken to test the validation of a POCT device for BTT. The study was conducted while maintaining strict criteria for blinding of test results among the interpreters for Gazelle as well as HPLC. However, molecular studies for the discordant cases could not be conducted and the results of HPLC were taken as final. There are conditions which can negatively affect the HbA2 levels other than iron deficiency like anaemia of chronic disease, juvenile myelomonocytic leukemia, sideroblastic anaemia, etc. On the other hand, HbA2 per cent is increased in α-gene triplication and megaloblastic anaemia. It was beyond the scope of the present study to assess for all these conditions among the participants.
Overall, the findings of this study suggest Gazelle to be good screening tool for BTT with high sensitivity, specificity and accuracy. Gazelle is already validated for detection homozygous sickle anaemia as well as sickle trait in various settings. Even though the Gazelle can flag the presence of any other haemoglobin variant like HbE and HbD in the sample, final confirmation of the diagnosis is required to be done by a diagnostic test like HPLC or CZE.
Financial support & sponsorship
None.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86:480-7.
- [Google Scholar]
- Disorders of haemoglobin: Genetics, pathophysiology and clinical management. J R Soc Med. 2001;94:602-3.
- [Google Scholar]
- Changing patterns in the epidemiology of β‐thalassemia. Eur J Haematol. 2020;105:692-703.
- [Google Scholar]
- Effectiveness of one tube osmotic fragility screening in detecting beta-thalassaemia trait. J Med Genet. 1981;18:266-70.
- [Google Scholar]
- Hemechip: An automated portable microchip electrophoresis platform for point-of-care sickle cell disease screening. Blood. 2017;130:3519.
- [Google Scholar]
- Paper based microchip electrophoresis for point-of-care haemoglobin testing. Analyst. 2020;145:2525-42.
- [Google Scholar]
- Integrated point-of-care device for anaemia detection and haemoglobin variant identification. IEEE Trans Biomed Eng 2019:37-40.
- [Google Scholar]
- STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Br Med J. 2015;277:826-32.
- [Google Scholar]
- Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med. 1996;3:895-900.
- [Google Scholar]
- First point-of-care diagnostic test for beta-thalassemia. Biosensors (Basel). 2022;14:83.
- [Google Scholar]
- Screening for beta-thalassaemia carriers in Egypt: significance of the osmotic fragility test. East Mediterr Health J. 2007;13:780-6.
- [Google Scholar]
- Molecular analysis of a thai beta-thalassaemia heterozygote with normal haemoglobin A2 level: Implication for population screening. Ann Clin Biochem. 2002;39:44-9.
- [Google Scholar]
- Validation of osmotic fragility test and dichlorophenol indophenol precipitation test for screening of thalassemia and Hb E. Southeast Asian J Trop Med Public Health. 2005;36:1538-42.
- [Google Scholar]
- Screening for the carriers of thalassemias and abnormal haemoglobins at the community level. Southeast Asian J Trop Med Public Health. 2002;33:145-50.
- [Google Scholar]
- Evaluation of single-tube osmotic fragility as a screening test for thalassemia. Am J Hematol. 2005;79:198-201.
- [Google Scholar]
- A simplified screening for alphathalassemia 1 (SEA type) using a combination of a modified osmotic fragility test and a direct PCR on whole blood cell lysates. Acta Haematol. 2002;108:74-8.
- [Google Scholar]
- Iron deficiency does not compromise the diagnosis of high HbA2 β thalassemia trait. Haematologica. 2012;97:472.
- [Google Scholar]
- Haemoglobin A2 lowered by iron deficiency and a-thalassemia: Should screening recommendation for b-thalassemia change? ISRN Hematol. 2013;2013:858294.
- [Google Scholar]
- Impact of iron deficiency on haemoglobin A2% in obligate β‐thalassemia heterozygotes. Int J lab Hematol. 2015;37:105-11.
- [Google Scholar]
- Elevated haemoglobin A2 as a marker for β-thalassemia trait in pregnant women. Tohoku J Exp Med. 2011;223:223-6.
- [Google Scholar]
- Erythrocyte indices in a large cohort of β‐thalassemia carrier: implication for population screening in an area with high prevalence and heterogeneity of thalassemia. Int J lab Hematol. 2019;41:513-8.
- [Google Scholar]
- Efficiency of premarital screening of beta-thalassemia trait using MCH rather than MCV in the population of Fars Province, Iran. Haematologia. 2002;32:129-33.
- [Google Scholar]
- P-054: Evaluation of first microchip-based point-of-care device for diagnosis of beta-thalassemia in India. Hemasphere. 2022;6:43.
- [Google Scholar]
- Evaluation of microchip-based point-of-care device “gazelle” for diagnosis of sickle cell disease in India. Front Med. 2021;8:639208.
- [Google Scholar]






