Translate this page into:
Incidence, antimicrobial susceptibility & out of pocket expenditure of severe enteric fever in Chandigarh, north India
For correspondence: Prof Madhu Gupta, Department of Community Medicine and School of Public Health, Post Graduate Institute of Medical Education & Research, Chandigarh 160 012, India e-mail: madhugupta21@gmail.com
-
Received: ,
Abstract
Background & objectives
Burden estimates of enteric fever are required to make policy decisions on introducing typhoid vaccine in India. Incidence, antimicrobial susceptibility, and out-of-pocket expenditure (OOPE) of enteric fever are estimated in Chandigarh, India.
Methods
A hybrid (facility and community-based) surveillance system was set up at a secondary care hospital to enrol patients above six months of age, hospitalized with fever, from a defined catchment population from May 2018 to March 2020. Blood samples were collected and cultured using an automated system (BD BACTECTM blood culture system). The Salmonella Typhi and S. Paratyphi isolates were characterized for antimicrobial susceptibility. OOPE was recorded after 14 and 28 days of discharge.
Results
Blood samples were collected from 97 per cent of the 1650 study participants enrolled. The incidence of enteric fever was 226.8 per 1,00,000 person-years (PY), severe typhoid fever 156.9 per 1,00,000 PY, and severe paratyphoid fever 69.9 per 1,00,000 PY. Salmonella was highly susceptible to ampicillin, azithromycin, and ceftriaxone (99.25%) and least susceptible to ciprofloxacin (11.3%). The OOPE due to hospitalization of individuals infected with S. Paratyphi [INR 8696.6 (USD 116)] was significantly higher than the individuals infected with S. Typhi [INR 7309 (USD 97.5), P=0.01], and among cases who were hospitalized for more than seven days [INR 12,251 (USD 163.3)] as compared with those with a stay of 3-7 days [INR 8038.2 (USD 107.2)] or less than three days [INR 5327.8 (USD 71), P<0.001].
Interpretation & conclusions
There was a high incidence of enteric fever, high OOPE, and resistance to ciprofloxacin.
Keywords
Enteric fever
epidemiology
incidence
OOPE
Salmonella
surveillance
typhoid
Enteric fever is a significant cause of morbidity and mortality across the globe, with an estimated annual burden of about 11,000,000 cases and 1,170,000 deaths1. India alone accounts for 82 and 75 per cent of this incidence and mortality in South Asia1. Recently, an estimated incidence of 108 to 970 cases per 100,000 person-years (PY) among 15 yrs of age or older and 12 to 1622 cases per 100,000 child-years among children between six months and 14 yr of age has been reported in a hospital-based surveillance system in India2. With the emergence of antibiotic-resistant strains such as the H58 haplotype in the 1990s, typhoid is becoming difficult to treat3. The emergence of extensively drug-resistant Salmonella Typhi in Pakistan highlights the need for urgent control measures4. A study conducted in five Asian countries reported a high cost of hospitalized cases of typhoid fever in Kolkata, India (USD 129) and Jakarta (USD 432)5.
Interventions focusing on improving water quality and sanitation, health education of the community, access to better diagnostics, effective typhoid vaccine, and treatment facilities could lead to the control of typhoid fever6. World Health Organization’s (WHO) position paper on typhoid vaccines recommends the use of typhoid conjugate vaccines (TCV) in children over six months of age in countries with a high burden of typhoid7. Community-based typhoid vaccination programmes in New Delhi in 2004 and Navi Mumbai in 2018 were reportedly effective in reducing typhoid infections8,9. However, to introduce vaccines in the national immunization programme, there is a further need to generate evidence around geographically representative data on the burden, severity, and cost of illness, which could guide the policymakers in making rational decisions10.
Surveillance programmes in many countries in Asia and Africa, such as Severe Typhoid Fever in Africa, Strategic Typhoid Alliance across Africa and Asia, Surveillance for Enteric Fever in Asia Program, and Surveillance for Enteric Fever in India (SEFI) with standard methodologies are established to measure the burden of enteric fever11. SEFI was a three-tier surveillance system established in India between 2017-2020. The first tier, estimates community-based enteric fever incidence through active surveillance at four sites2. The second tier, in secondary care hospitals with a defined catchment population, reported the incidence of severe enteric fever cases at six sites via hybrid methodology2. The third tier was established at eight tertiary care hospitals to examine the antimicrobial susceptibility patterns and spectrum of clinical severity in culture-confirmed enteric fever cases. In this study, we present the incidence of enteric fever, antimicrobial susceptibility patterns, and cost of illness in Chandigarh under the second-tier surveillance system.
Material & Methods
A facility-based surveillance and community-based healthcare utilization survey (HCUS) hybrid was conducted from May 2018 to March 20202 at Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh after obtaining approval from the Institutional Ethics Committee and Chandigarh Administration. After sharing the participant information sheet, written informed consent was obtained from the study participants/legal guardians. A written assent was obtained from the children aged seven to 18 yr.
I. Facility-Based Surveillance System:
Study area and health facility
The surveillance was set up in a secondary care, 50-bedded Civil Hospital, Chandigarh, where >75 per cent of individuals with febrile illness were admitted from the catchment area (a mix of urban and urbanized village) with a population of 1,43,000.
Study population
Individual aged six months and above from the catchment area admitted to the study hospital (emergency/medicine/paediatrics/obstetrics and gynecology ward) for more than six hours with acute febrile illness (temperature >98.4°F) qualified to be included in the study. Study participants with positive blood cultures for Salmonella Typhi or Salmonella Paratyphi (A/B/C) were considered confirmed severe typhoid or paratyphoid fever cases2,11. We enrolled 273 paediatric cases (< 15 yr) and 1377 adults (≥15 yr). Due to the non-availability of a paediatrician between January 2019 and March 2020 in the study hospital, 67 paediatric cases from the catchment area were enrolled in the referral hospitals.
Data collection methods and tools
The surveillance team, comprising a medical officer, public health officer, nurses, field investigators, and laboratory technicians, was trained by community and internal medicine experts. The eligible individuals, identified by screening the admission registers in casualty/wards, were approached; consent obtained, matched with the inclusion and exclusion criteria and recruited consecutively. A pretested predesigned structured interview schedule was used to collect information regarding socio-demographic profile, clinical signs and symptoms, and medical history and laboratory investigations related to the current episode of illness by face-to-face interviews. (Supplementary Material I). Hospital records/treatment charts were used to retrieve information on the course of illness and treatment, including antibiotics administered during hospitalization. The culture-confirmed enteric fever individuals were recontacted telephonically or by visiting their homes on the 14th and 28th day of discharge to collect information regarding the course of illness after discharge using a recontact form (Supplementary Material II). Data regarding out-of-pocket expenditure (OOPE) on the cost of illness, including direct medical and non-medical, and the indirect costs per day in the hospital, days 14 and 28 of admission, was collected for culture-confirmed enteric fever cases using a pretested and predesigned questionnaire (Supplementary Material III). The detailed methodology of the cost of illness due to severe enteric fever is presented elsewhere12.
Laboratory procedures
About 1-3 ml blood sample was obtained from the infants, 3-5 ml from study participants of age 1-15 yr, and 8-10 ml from adults (≥15 yr) to ensure the adequacy of blood volume for better sensitivity13,14.The blood samples were cultured in the department of Medical Microbiology, PGIMER, Chandigarh, using an automated system (BD BACTECTM FX40 blood culture system)15. S. Typhi and S. Paratyphi isolates were characterized for antimicrobial susceptibility patterns using the disc diffusion method16. The isolates were stored in nutrient-deep agar in the Department of Microbiology, PGIMER, at -80°C before shipping to Central Laboratory, Christian Medical College (CMC), Vellore, for further genomic characterization. The susceptibility patterns of Salmonella were assessed against ampicillin, azithromycin, ceftriaxone, chloramphenicol, ciprofloxacin, cotrimoxazole, and nalidixic acid. These drugs were used routinelyduring antimicrobial susceptibility testing per the standard operating procedures. Nalidixic acid was not used to treat the study participants because of its reported resistance against Salmonella17,18.
Data quality assurance
The study coordinator (AB) visited the study sites thrice a week and crosschecked all the forms with the source documents. The principal investigator (MG) supervised weekly and crosschecked 10 per cent of the forms filled. The external monitoring team from CMC, Vellore, supervised six monthly and randomly crosschecked 10 per cent of the forms. The internal and external team maintained quality control of the blood sample collection, storage, and transportation to the study laboratory as per standard operating procedures.
II. Healthcare Utilization Survey (HCUS)
HCUS was conducted in 2019 by an independent agency recruited by the CMC, Vellore, to estimate the proportion of all febrile hospitalizations from the catchment population in the study hospital19. This was achieved through household surveys in the catchment area, in which the details on household socio-economic status, individual demographics, illness episodes based on a 2 wk recall, hospitalization events based on a six month recall, and deaths that occurred over the past year were collected.
Data analysis
Statistical analysis was done using Statistical Package for Social Sciences version 21 (SPSS Inc., IBM Corp, Chicago, Armonk, NY). The crude incidence of severe enteric fever was estimated by dividing the number of culture-confirmed typhoid and paratyphoid fevers by the catchment population (2019-2020) and adjusting for the surveillance period. Crude incidence of typhoid and paratyphoid fever was calculated per lakh person-years (population x time spent by a participant in the study). Based on the results of HCUS, the incidence of enteric fever was adjusted using a multiplier, i.e., the reciprocal of the proportion of febrile illness hospitalizations from the catchment areas at the surveillance hospital (A1), compliance to the study protocol (A2), and poor sensitivity of blood culture using an adjustment based on blood volume inoculated in culture (A3)2. The correction for blood culture sensitivity was assumed to be 60 per cent, with an uncertainty range from 50-70 per cent20. The adjusted incidence was calculated as follows: Crude Incidence/A1*A2*A3. The quantitative data was analyzed using the student’s t test, and the ordinal data was analyzed using the Mann-Whitney U Test. Multiple logistic regression analysis was done to understand the relationship between the different confounding variables and the positivity of blood cultures for enteric fever. The costing information for all the culture-confirmed enteric fever cases was presented in terms of mean expenditure and standard deviation.
Results
Of the 2652 febrile admissions in the study hospitals during the study period, 1650 (62.2%) individuals met the eligibility criteria. As blood samples were obtained from 1600 (97%) study participants, including 244 (15.25%) paediatric and 1356 (84.7%) adult samples, these were enrolled. The proportion of blood samples collected on day one of admission was 85 per cent, on day two 14 per cent, and beyond day two was one per cent. The mean days of illness at which the blood sample was collected was 6.23. The mean blood volume was 9.36 ml for the age group >15 yr and 7.2 ml for the age group <15 yr. The most common pathogen found in the blood culture was S. Typhi (n=92; 5.75%), followed by S. Paratyphi (n=41; 2.56%), Escherichia Coli (n=5; 0.3%) and Staphylococcus (n=4; 0.25%). The seasonal trend of S. Typhi and S. Paratyphi is shown in Figure.
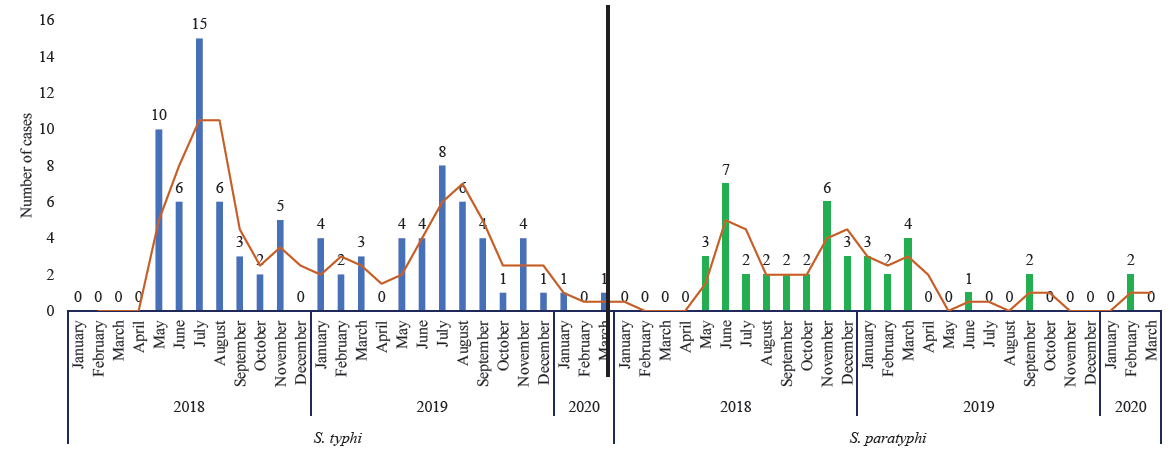
- Year-wise and Month-wise trend of culture-confirmed enteric fever cases at study hospitals.
There was a significantly (P value=0.046) higher proportion of males among enteric fever cases (63.9%) as compared to non-enteric fever cases (54.9%) and the paediatric age group (22.5%) was more affected among them as compared to non-enteric fever cases (16%) (Table I). Significant risk factors of culture-confirmed enteric fever cases were being a male [Adjusted odd ratio (AOR) 1.4, 95% confidence interval (CI): 1.01, 2.1, P=0.046], age <15 yr (AOR 1.5, 95% CI: 0.99, 2.34, P=0.03) and number of days between the onset of illness and admission to the study hospital of more than three days (AOR 2.37, 95% CI: 1.22,4.6, P-0.01) (Table II). The clinical features of the enrolled participants are given in Supplementary Table I. More than 90 per cent of the isolates of S. Typhi and S. Paratyphi were found to be sensitive to ampicillin, azithromycin, chloramphenicol, ceftriaxone, cotrimoxazole, except ciprofloxacin (9-11%) (Table III). The adjusted incidence of severe typhoid fever was 156.9 per 100,000 PY (95% CI: 127.8-192.4), and severe paratyphoid fever was 69.9 per 1,00,000 PY (95% CI: 51.5-94.95) (Table IV).
| Characteristics |
EF positive n=133 (%) |
EF negative n=1517 (%) |
Total AFI N=1650 (%) |
P Value |
|---|---|---|---|---|
| Gender | ||||
| Male | 85 (63.9) | 833 (54.9) | 918 (55.6) | 0.046 |
| Female | 48 (36.01) | 684 (45.08) | 732 (44.4) | |
| Age | ||||
| Paediatrics (<15 yr) | 30 (22.5) | 243 (16.01) | 273 (16.5) | 0.051 |
| Adult (≥15 yr) | 103 (77.4) | 1274 (83.9) | 1377 (83.5) | |
| Mean age (SD) | 21.8 (11.12) | 28.4 (15.7) | - | <0·0001 |
| Highest education of any family member in the household | ||||
| Illiterate | 2 (1.5) | 35 (2.3) | 37 (2.2) | 0.25 |
| Primary | 9 (6.8) | 116 (7.6) | 125 (7.6) | |
| Middle | 29 (21.8) | 317 (20.9) | 346 (21) | |
| Secondary | 26 (19.5) | 255 (16.8) | 281 (17) | |
| Higher secondary | 19 (14.3) | 344 (22.7) | 363 (22) | |
| Diploma/degree & above | 48 (36.1) | 450 (29.7) | 498 (30.2) | |
| Highest occupation of any family member in the household | ||||
| Business | 31 (23.3) | 273 (16.5) | 304 (18.42) | 0.44 |
| Daily wage | 31 (23.3) | 340 (20.6) | 371 (22.4) | |
| Household work | - | 10 (0.6) | 10 (0.6) | |
| Salaried | 71 (53.6) | 890 (53.9) | 961 (58.24) | |
| Unemployed | - | 4 (0.24) | 4 (0.2) | |
| Type of house | ||||
| Hut/govt house | - | 4 (0.2) | 4 (0.2) | 0.46 |
| Kutcha | 30 (1.9) | 30 (1.8) | ||
| Mixed house | - | 20 (1.3) | 20 (1.2) | |
| Pucca house | 133 (100) | 1463 (96.4) | 1596 (96.7) | |
Association of 6 socio-demographic characteristics with culture confirmation of enteric fever. AFI, all febrile illness; EF, enteric fever; SD, standard deviation; govt, government
| Demographics factors | Adjusted OR (95% CI) | P value |
|---|---|---|
| Sex | ||
| Female | Ref | _ |
| Male | 1.4 (1.006, 2.101) | 0.046 |
| Age category | ||
| Adult (≥15 yr) | Ref | |
| Pediatrics (<15 yr) | 1.5 (0.99, 2.34) | 0.03 |
| Number of days between onset of illness & admission to study hospital | ||
| Same day | 0.65 (0.13, 3.04) | 0.58 |
| 1 day | 0.606 (0.2, 1.8) | 0.36 |
| 2 days | Ref | |
| ≥3 days | 2.37 (1.22, 4.604) | 0.011 |
| Highest education of any family member in the household | ||
| Illiterate | Ref | |
| Primary | 1.7 (0.42, 7.8) | 0.4 |
| Middle | 1.3 (0.28, 6.5) | 0.7 |
| Secondary | 1.6 (0.36, 6.9) | 0.53 |
| Higher secondary | 0.96 (0.21, 4.3) | 0.96 |
| Diploma/degree & above | 1.86 (043, 8) | 0.4 |
| Highest Occupation of any family member in the household | ||
| Business | Ref | |
| Daily wage | 1.2 (0.72, 2.1) | 0.41 |
| Household work | - | |
| Salaried | 1.42 (0.9, 2.2) | 0.11 |
| Unemployed | - | |
Dependent, enteric fever positive & negative cases; Independent, demographic variables. OR, odds ratio; CI, confidence interval
| Drug |
Salmonella Typhi n=92 (%) |
Salmonella Paratyphi n=41 (%) |
Total n=133 (%) |
Sensitivity (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | S | I | R | ||
| Ampicillin | 91 (98.9) | 0 | 1 (1.1) | 41 (100) | 0 | 0 | 132 (99.3) | 0 | 1 (0.7) | 99.3 |
| Azithromycin | 91 (98.9) | 0 | 1 (1.1) | 41 (100) | 0 | 0 | 132 (99.3) | 0 | 1 (0.7) | 99.3 |
| Ceftriaxone | 90 (98.9) | 0 | 2 (2.2) | 41 (100) | 0 | 0 | 131 (98.4) | 1 (0.7) | 0 | 98.4 |
| Chloramphenicol | 91 (98.9) | 1 (1.1) | 0 | 41 (100) | 0 | 0 | 132 (99.3) | 1 (0.7) | 0 | 99.3 |
| Ciprofloxacin | 9 (9.8) | 70 (76.1) | 11 | 6 (11.3) | 32 | 3 (5.7) | 15 (11.3) | 70 | 14 | 11.3 |
| Cotrimoxazole | 89 (96.7) | 1 (1.1) | 2 (2.2) | 41 (100) | 0 | 0 | 130 (97.7) | 1 (0.7) | 2 (1.4) | 97.7 |
| Nalidixic acid | 3 (3.3) | 2 (2.2) | 87 (94.5) | 1 (2.4) | 2 (4.8) | 38 (92.7) | 4 (2.8) | 2 (1.4) | 122 (91.7) | 2.8 |
S, susceptibility; I, intermediate; R, resistant
| Age group | Person year | Culture confirm method | Crude incidence | Study facility utilization A1 | Compliance to the protocol A2 | Blood culture sensitivity A3 | Adjusted incidence (Crude Incidence/A1*A2*A3) | 95% CI |
|---|---|---|---|---|---|---|---|---|
| Salmonella Typhi | ||||||||
| Paediatrics (<15 yr) | 71,595 | 21 | 29.3 | 0.22 | 0.88 | 0.6 | 252.2 | 164.68-347.22 |
| Adult (≥15 yr) | 1,93,571 | 71 | 36.7 | 0.42 | 0.99 | 0.6 | 147.02 | 116.52-185.5 |
| Overall | 2,65,164 | 92 | 34.7 | 0.38 | 0.97 | 0.6 | 156.9 | 127.8-192.43 |
| Salmonella Paratyphi | ||||||||
| Paediatrics (<15 yr) | 71,595 | 9 | 12.6 | 0.22 | 0.88 | 0.6 | 108.2 | 55.95-207.98 |
| Adult (≥15 yr) | 1,93,571 | 32 | 16.5 | 0.42 | 0.99 | 0.6 | 66.2 | 46.85-93.71 |
| Overall | 2,65,164 | 41 | 15.5 | 0.38 | 0.97 | 0.6 | 69.9 | 51.5-94.95 |
The mean OOPE incurred on treatment of study participants infected with S. Paratyphi [INR 8696.6 (USD 115.9)] was significantly higher than the study participants having infection with S. Typhi [INR 7309 (USD 97.5), P=0.01]. The mean OOPE due to hospitalization of adult study participant [INR 8393.6 (USD 111.9)] was significantly higher as compared to the paediatric affected individuals [INR 5481.4 (USD 73.1), P<0.001] (Table V).
| Characteristics | Enteric fever cases, INR (USD), (n=133) | P value | ||
|---|---|---|---|---|
| Type | S. Typhi (n=92), n(%) | S. Paratyphi (n=41), n(%) | ||
| Total family income | 20,739 (276.5) | 33,244 (443.3) | 0.084 | |
| Total direct expenditure | 4234.5 (56.5) | 4475.7 (59.7) | 0.245 | |
| Total indirect expenditure | 3074.5 (41) | 4220.9 (56.3) | 0.015 | |
| Total expenditure | 7309 (97.5) | 8696.6 (115.9) | 0.01 | |
| Age group | Paediatric (<15 yr) (n=30) | Adult (> 15 yr) (n=103) | ||
| Total direct expenditure | 4224.3 (56.3) | 4333.5 (57.8) | 0.22 | |
| Total indirect expenditure | 1257.1 (16.8) | 4060.2 (54.1) | <0.001 | |
| Total expenditure | 5481.4 (73.1) | 8393.6 (111.9) | <0.001 | |
| Gender | Male (n=84) | Female (n=49) | ||
| Total direct expenditure | 4127.6 (55) | 4619.6 (61.6) | 0.32 | |
| Total indirect expenditure | 4150.8 (55.3) | 2188.7 (29.2) | <0.001 | |
| Total expenditure | 8278.4 (110.4) | 6808.2 (90.8) | 0.03 | |
| Duration of hospitalization | Upto 3 days (n=35) | 3-7 days (n=85) | >7 days (n=13) | |
| Total direct expenditure | 2842.5 (37.9) | 4704 (62.7) | 5672.8 (75.6) | <0.001 |
| Total indirect expenditure | 2485.4 (33.1) | 3334.2 (44.5) | 6578.1 (87.7) | <0.001 |
| Total expenditure | 5327.8 (71) | 8038.2 (107.2) | 12251 (163.3) | <0.001 |
| Outcome of hospitalization | Recovered without complication (n=113) | Sequalae/death/referred) (n=19) | ||
| Total direct expenditure | 3952.2 (52.7) | 6532.5 (87.1) | <0.001 | |
| Total indirect expenditure | 3476.7 (46.4) | 3141 (41.9) | 0.11 | |
| Total expenditure | 7428.9 (99.1) | 9673.5 (129) | <0.001 | |
Discussion
The estimated incidence of 226.8 per 100,000 PY in this investigation was in line with the study conducted by the Global Burden of Disease collaborators in 20171. The reasons for such high incidence in Chandigarh could be the poor hygienic conditions in the study area (urbanized village) leading to higher chances of fecal-oral transmission of the disease; the better health-seeking behavior of the study population and preference to utilize the study hospitals due to their proximity; better detection rates in the blood cultures as these were processed in laboratory at the department of Medical Microbiology, PGIMER, accredited by National Accreditation Board for Testing and Calibration Laboratories (NABL). The culture positivity rate in this study (8.06%) was comparable to the study done by Sharma et al21 in north India (10.8%). The seasonal trend observed in our study was similar to that observed in a time series analysis of the Integrated Disease Surveillance Program’s data of the Government of India from 2014 to 2017 in Chandigarh22.
The higher incidence of typhoid and paratyphoid fever among children (<15 yr), especially school children and males, aligned with the findings of an earlier study in the region23 and is possibly linked to eating outside food. The symptoms reported by the study participants were consistent with the clinical features reported by other studies across India24. The complications reported in our study were similar but less (5%) than those reported in previous studies25. The mean hospitalization (4 days; range up to 2 wk) of enteric fever cases in this study was in line with a previous study26.
The antimicrobial susceptibility patterns aligned with the patterns reported by other investigators, where S. Typhi and S. Paratyphi were 100 per cent sensitive to ceftriaxone but resistantto ciprofloxacin23. The cause of such resistance against the first line of drugs could be due to overuse of fluoroquinolones over the last decade27. The re-emergence of sensitivity to drugs like chloramphenicol might be attributed to the restricted use of chloramphenicol because of the risk of bone marrow suppression.
This study’s OOPE estimates exceeded that of the Kolkata study5 (2004), conducted one and a half decades ago. The Surveillance for Enteric Fever in Asia Project (2016–2018) studies reported a slightly lower OOPE, as they excluded medical costs for non-enteric fever patients28. India has one of the world’s highest over-the-counter antibiotic sales contributing to growing antibiotic resistance29 and could lead to higher estimates of out-of-pocket expenditure30. A decision analytic modelling study recommended that the use of typhoid conjugate vaccine in the community will reduce mortality and lead to a significant gain in an incremental cost per quality-adjusted life year gained31.The study by Kumar et al12 (2021) reported the mean cost obtained from all the secondary (tier 2) and tertiary care hospitals (tier 3). We reported Chandigarh-specific estimates as it had the highest culture-confirmed enteric fever cases and represented an urban area (all other sites were rural). However, the total direct and indirect OOPE reported by Kumar et al12 (2021) was higher than that reported in this study because cases were managed in public sector hospitals. In contrast, the study by Kumar et al12 included the cost of 50 per cent of patients being managed at private/charity hospitals with higher OOPE.
The superior sensitivity and specificity of blood culture to that of rapid diagnostic tests like Typhidot IgM led to its preference for detecting bacteremia in this study32. As the volume of blood collected is an important predicting factor for getting positive results by blood culture, it is emphasized in this study to detect culture-confirmed typhoid cases13,14. If conducted alone, facility-based surveillance would underestimate the disease burden in a community, as not all cases from the catchment would visit that facility. In the hybrid model, the crude incidence rate estimated by facility-based surveillance is adjusted for febrile hospitalizations from the catchment in facilities other than study hospitals33. This design represents a strategy for sustainable population-referenced disease burden estimations in low-resource settings like India.
The consecutive recruitment of eligible study participants in the study hospital might be affected by the seasonality, limiting the generalizability of the findings. To address this issue, the surveillance was extended for more than one year (May 2018 to March 2020), and a hybrid surveillance system was used to estimate the incidence. While the healthcare utilization survey had adjusted this rate, we might have missed some proportion of the population in this age group. The absence of a full-time paediatrician in the study hospitals excluded potential paediatric cases who might not have sought care there. The paediatric patients from the catchment area enrolled from the referral tertiary care institute might had prior antibiotic consumption, resulting in negative blood culture results. Information on the OOPE on medicines available over the counter was not collected, which might have increased the estimated of OOPE. Although data on water, sanitation and hygiene (WASH) practices were not collected, as the study was conducted primarily in urbanized villages with poor sanitary conditions, the WASH practices were assumed to be compromised.
Overall, this study provided evidence of the high incidence of typhoid disease, OOPE and the emergence of resistance to ciprofloxacin among the population aged six months and above residing mainly in urbanized villages with poor socio-economic, housing, and sanitary conditions in Chandigarh, north India. This evidence might help policymakers implement strategies, such as promoting WASH practices, introducing a typhoid conjugate vaccine, and improving point-of-care diagnostic tests at the primary healthcare level for an early diagnosis of enteric fever. Strengthening existing surveillance systems at the primary, secondary, and tertiary care levels, including the Integrated Disease Surveillance Program (IDSP), is crucial for continuously monitoring typhoid disease incidence and assessing the long-term impact of these interventions.
Acknowledgment
The authors acknowledge the study team who coordinated the data collection, the team at Christian Medical College, Vellore for support, and the participants and their families for taking part in the study.
Financial support & sponsorship
This study was funded by a grant from the Bill and Melinda Gates Foundation (grant INV-009497-OPP1159351) and The Fogarty International Center, National Institutes of Health, USA through D43 TW007392.
Conflicts of Interest
None.
Use of Artificial Intelligence (AI)-Assisted Technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- The global burden of typhoid and paratyphoid fevers: A systematic analysis for the global burden of disease study 2017. Lancet Infect Dis. 2019;19:369-81.
- [Google Scholar]
- The coalition against typhoid: Mobilizing a community for a global fight. Clin Infect Dis. 2019;68:S161-4.
- [Google Scholar]
- Extensively drug-resistant typhoid — are conjugate vaccines arriving just in time? N Engl J Med. 2018;379:1493-5.
- [Google Scholar]
- Cost of illness due to typhoid fever in five Asian countries. Trop Med Int Health. 2011;16:314-23.
- [Google Scholar]
- Principles and considerations for adding a vaccine to a national immunization programme: from decision to implementation and monitoring. Available from: https://apps.who.int/iris/handle/10665/111548, accessed on August 23, 2022.
- Typhoid vaccines: WHO position paper, March 2018 - Recommendations. Vaccine. 2019;37:214-16.
- [Google Scholar]
- Community-based typhoid vaccination program in New Delhi, India. In: Eighth International Conference on Typhoid Fever and Other Invasive Salmonelloses. 2013. March 1-2
- [Google Scholar]
- Programmatic effectiveness of a pediatric typhoid conjugate vaccine campaign in Navi Mumbai, India. Clin Infect Dis. 2023;77:138-44.
- [Google Scholar]
- The burden of typhoid and paratyphoid in India: Systematic review and meta-analysis. PLoS Negl Trop Dis. 2016;10:e0004616.
- [Google Scholar]
- The surveillance for enteric fever in Asia project (SEAP), severe typhoid fever surveillance in Africa (SETA), surveillance of enteric fever in India (SEFI), and strategic typhoid alliance across Africa and Asia (STRATAA) population-based enteric fever studies: A review of methodological similarities and differences. Clin Infect Dis. 2020;71:S102-10.
- [Google Scholar]
- Cost of illness due to severe enteric fever in India. J Infect Dis. 2021;224:S540-7.
- [Google Scholar]
- The relationship between blood sample volume and diagnostic sensitivity of blood culture for typhoid and paratyphoid fever: A systematic review and meta-analysis. J Infect Dis. 2018;218:S255-67.
- [Google Scholar]
- Factors predicting blood culture positivity in children with enteric fever. J Infect Dis. 2021;224:S484-93.
- [Google Scholar]
- BD. BD BACTEC™ FX40 Instrument. Available from: https://www.bd.com/en-us/products-and-solutions/products/product-page.442296, accessed on May 19, 2022.
- Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol. 2009;15:55-63.
- [Google Scholar]
- Clinical outcomes in typhoid fever: Adverse impact of infection with nalidixic acid-resistant Salmonella typhi. BMC Infect Dis. 2005;5:1-0.
- [Google Scholar]
- Healthcare utilization survey in the hybrid model of the Surveillance for Enteric Fever in India (SEFI) study: Processes, monitoring, results, and challenges. J Infect Dis. 2021;224:S529-39.
- [Google Scholar]
- Burden of typhoid fever in low-income and middle-income countries: A systematic, literature-based update with risk-factor adjustment. Lancet Glob Health. 2014;2:e570-80.
- [Google Scholar]
- Seroprevalence of Salmonella typhi in a tertiary care facility in North India. Int J Curr Microbiol Appl Sci. 2015;4:946-9.
- [Google Scholar]
- Comparison of time series models predicting trends in typhoid cases in Northern India. Southeast Asian J Trop Med Public Health. 2019;50:347-56.
- [Google Scholar]
- Epidemiological profile and antimicrobial resistance pattern of enteric fever in a tertiary care hospital of North India–A seven year ambispective study. Acta Medica. 2019;61:125-30.
- [Google Scholar]
- A study on clinical profile of typhoid fever at Government General Hospital, Nizamabad, Telangana, India. Int J Contemp Pediatr. 2019;6:2642.
- [Google Scholar]
- A retrospective review of hospital-based data on enteric fever in India, 2014–2015. J Infect Dis. 2018;218:S206-13.
- [Google Scholar]
- A prospective observational evaluation of the clinical and laboratory profiles of typhoid fever in children in the Bihar region of India. Eur J Mol Clin Med. 2021;8:1377.
- [Google Scholar]
- Changing trends of culture-positive typhoid fever and antimicrobial susceptibility in a tertiary care North Indian Hospital over the last decade. Indian J Med Microbiol. 2018;36:70-6.
- [Google Scholar]
- Typhoid and paratyphoid cost of illness in Nepal: Patient and health facility costs from the surveillance for enteric fever in Asia project II. Clin Infect Dis. 2020;71:S306-18.
- [Google Scholar]
- Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: An analysis of pharmaceutical sales data. Lancet Infect Dis. 2021;21:107-15.
- [Google Scholar]
- Quantifying the financial burden of households’ out-of-pocket payments on medicines in India: A repeated cross-sectional analysis of National Sample Survey data, 1994–2014. BMJ Open. 2018;8:e018020.
- [Google Scholar]
- What proportion of Salmonella Typhi cases are detected by blood culture? A systematic literature review. Ann Clin Microbiol Antimicrob. 2016;15:1-8.
- [Google Scholar]
- Integrating facility-based surveillance with healthcare utilization surveys to estimate enteric fever incidence: Methods and challenges. J Infect Dis. 2018;218:S268-76.
- [Google Scholar]






