Translate this page into:
Exploratory analysis of serum Krebs von den Lungen-6, blood gas analysis & Brixia score in determining COVID-19 severity & mortality
For correspondence: Dr Muhammad Amin, Department of Pulmonology and Respiratory Medicine, Faculty of Medicine, Universitas Airlangga Hospital, Surabaya, East Java 60115, Indonesia e-mail: muh.amin@fk.unair.ac.id
-
Received: ,
Abstract
Background & objectives
Krebs von den Lungen-6 (KL-6) is primarily expressed by the damaged type II pneumocytes. In this context, the relationship of KL-6 with blood gas analysis (BGA) parameters and Brixia score is still limitedly discussed. This study aims to analyze the correlation of KL-6, BGA and Brixia scores to the severity and mortality of COVID-19.
Methods
A cross-sectional study was conducted in adult COVID-19 positive individuals at Universitas Airlangga Hospital, Surabaya, East Java, Indonesia, from March to August 2021. KL-6, BGA, and Brixia scores were compared according to severity (severe vs. non-severe) and mortality (non-survivor vs. survivor). The receiver operating characteristic (ROC) analysis was also performed to define the optimal cut-off, sensitivity, as well as the specificity of KL-6, BGA and Brixia scores to determine the COVID-19 severity and mortality.
Results
Total 35 severe and 20 non-severe COVID-19 positive individuals were enrolled in this study. Of those, there were 22 non-survivors. No significant difference in serum KL-6 levels was observed in the severity and mortality groups. KL-6 and HCO3– had positive correlation in the severe group (r=0.37). KL-6 and Brixia scores showed a significant negative correlation among COVID-19 positive individuals (r=–0.283; P=0.036). KL-6 and Brixia scores together served as the best severity markers in the current study [AUC 0.809 (0.697–0.920); Sn/Sp=0.686/0.900)], followed by KL-6 and P/F ratio [AUC 0.800 (0.637–0.963); Sn/Sp=0.971/0.750].
Interpretation & conclusions
The findings of this study suggest that KL-6 has the potential to be a useful adjunct laboratory parameter to the BGA and Brixia score representing COVID-19 severity and mortality.
Keywords
Biomarker
blood gas analysis
brixia
COVID-19
KL-6
mortality
SARS-CoV-2-severity
Coronavirus disease 2019 (COVID-19) is a pandemic viral infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The associated clinical spectrum varies, ranging from asymptomatic to critical cases, with a case fatality rate of 6.5 per cent, or even higher to 9.1 per cent in Indonesia1,2. During the disease progression, 20.7–31.4 per cent reportedly may develop into severe cases and 4.9–11.5 per cent may need ventilatory support3 in context of this disease progression many biomarkers are still needed to explore the severity and mortality of COVID-19, including Krebs von den Lungen-6 (KL-6) and blood gas analysis (BGA). KL-6 (MUC1 or Mucin-1) is a sialoglycoprotein expressed by damaged type II alveolar cells1,4. SARS-CoV-2 replication has been shown to increase serum KL-6 levels, representing the damaged lung area5,6. Circulating KL-6 in the the bloodstream can induce inflammatory processes, which may lead to organ failure and mortality1,5,6. Furthermore, it is thought that SARS-CoV-2 infection downregulate its own port entry, through the angiotensin-converting enzyme 2 (ACE2)7. This process disrupts the acid-base equilibrium by upregulating the classic renin-angiotensin system (RAS) pathway. It interferes with the renal balancing mechanism, which can be reflected in the BGA results7. Silent hypoxemia, fever, multi-organ inflammation, thrombogenesis and carotid body suppression may disturb the pulmonary vasculature eventually leading to alveoli or damage through acute respiratory distress syndrome (ARDS)8.
Radiologic assessments are also vital in determining COVID-19 severity and mortality. Brixia score, a semi-quantitative assessment, is a simple assessment of the chest X-ray (CXR)-based scoring in COVID-199. This scoring is designed to rank the pulmonary involvement area and characteristics through a total 18-point severity scale9,10. The CXR is divided into upper, middle and lower zones for each lung, then scored 0–3 for each zone, with 0 as ‘no lung abnormalities’ and 3 as ‘visible interstitial and alveolar infiltrate’9.
Due to the quick deterioration of COVID-19, some laboratory parameters and radiological assessments are essential to determine its severity and mortality. Furthermore, with a hypothesis that KL-6, BGA and Brixia scores correlate with each other and the COVID-19 severity and mortality it was envisioned that such a study could provide novel knowledge regarding its pathophysiology. Currently, no study discusses their relationship with COVID-19. Hence, this study aimed to analyse the relationship between KL-6, BGA and Brixia scores in the severity and mortality of Indonesian COVID-19 positive individuals in Indonesia. Their sensitivity and specificity were also determined.
Material & Methods
Study design and setting
This cross-sectional study was conducted on adult hospitalized COVID-19 positive individuals enrolled from March to August 2021 at Universitas Airlangga Hospital, Surabaya, Indonesia. All clinical and laboratory data were collected from hospital medical records. A blood examination was conducted at the same hospital. This study was approved by the ethics committee of Universitas Airlangga Hospital.
Eligibility criteria
The inclusion criteria in this study: (i) adults ≥18 yr, COVID-19 positive confirmed by reverse transcription polymerase chain reaction (RT-PCR) of nasopharyngeal swab; (ii) undergoing CXR examination and evaluated for Brixia score; (iii) individuals with KL-6 and BGA results; and (iv) participants who provided their written informed consents. The exclusion criteria were individuals with pre-existing or history of lung tuberculosis (TB), interstitial lung disease (ILD) and/or chronic obstructive pulmonary disease, pregnant women and individuals with human immunodeficiency virus infection.
Clinical data and laboratory assessment
Age, gender, length of stay, respiratory rates (RR) and comorbidities were recorded as the clinical data. The Charlson Comorbidity Index (CCI) were calculated and classified them into three groups based on the scores: 0–1; 2–3; and ≥411. Complete blood count, liver function test, renal function test, electrolyte, anion gap, D-dimer, ferritin, interleukin-6 (IL-6), procalcitonin (PCT), C-reactive protein (CRP), KL-6, BGA and PaO2/FiO2 ratio (P/F ratio) were also collected. KL-6 were measured using the Bioassay Technology Laboratory KL-6 ELISA reagent kit (BT-Lab, Cat. No E1980Hu) and interpreted using iMarkTM Microplate Absorbance Reader (Bio-Rad Laboratories Inc., Hercules, CA). CXR was scored using the Brixia system12,13, first interpreted by a radiologist followed by a pulmonologist and then by the senior radiologist to confirm the interpretation. The Brixia score results were then classified into four groups of severity: score 0 as normal; score 1–6 as mild; score 7–12 as moderate; and score 13–18 as severe13.
The study participants were categorized into four severity levels based on the Indonesian COVID-19 guideline. Those who had any signs and/or symptoms of COVID-19 without any proof of pneumonia or hypoxia were grouped as ‘mild’; those who had pneumonia proven by clinical assessment or imaging with SpO2≥93% on room air at sea level were grouped as ‘moderate’; those who had severe pneumonia indicated by RR >30 breaths/min, severe respiratory distress, or SpO2<93% on room air at sea level were grouped as ‘severe’; and those who had ARDS, sepsis and/or, septic shock were grouped as critical13,14. The severity classification was simplified into non-severe (mild and moderate) and severe (severe and critical). Furthermore, samples were also classified into survivors and non-survivors.
Statistical analysis
The results were presented as mean±standard deviation (SD) and median (interquartile range [IQR]). We analyzed the comparison between groups, either severe vs. non-severe or non-survivor vs. survivor. Mann–Whitney U and Chi-square tests were performed for ordinal and nominal data, respectively. Fisher’s exact test was also performed if the conditions of Chi-square test were not met. For the continuous data, an independent t-test or Mann–Whitney U test was applied depending on the Shapiro–Wilk normality test. An independent t-test was selected if P ≥0.05; otherwise, the Mann–Whitney U test was chosen. Pearson’s correlation was performed to assess the correlation of parameters. We also analysed the receiver operating characteristics (ROCs) curve and its components, including area under the curve (AUC), sensitivity, specificity and optimal cut-off (Youden’s index). Statistical analyses were conducted using IBM SPSS Statistics version 25.0 (SPSS Inc., Chicago, IL). P<0.05 was considered significant. Furthermore, uni- and multivariate logistic regressions were performed to obtain odds ratios (ORs) if P<0.05. Missing values were imputed by aggregating five iterations of multiple imputations by chained equations imputation method into their mean values15. Multiple imputations were only conducted if the missing values on a variable were <5% to avoid result bias16.
Results
A total of 55 COVID-19 positive individuals were included (49.1% were male) and their severity and mortality parameters were compared (Table I and Supplementary Table I). The RR was significantly different in the severe group (30.5±5.1 vs. 24.2±3.5; P<0.001) and in non-survivors (30.7±5.7 vs. 26.6±4.8; P=0.005) compared to each opposing group. Multivariate logistic regression (Table II) showed that RR was significantly higher in the severe group (OR 1.567 [1.130–2.174]; P=0.007), but not in the non-survivors (OR 1.094 [0.904–1.325; P=0.355) as compared to each opposing group. The two most common comorbidities in this study were diabetes mellitus and hypertension (each 18.2%). In the CCI, a score of 0-1 was the most common among all participants (63.6%). However, none of the groups showed significantly different numbers of comorbidities and CCI scores. Missing values were present for D-dimer, ferritin, IL-6, PCT and CRP (Supplementary Table II). A significantly higher platelet (P=0.011) and D-dimer (P=0.002) levels were observed in the severe groups. Red blood cells, serum glutamic oxaloacetic transaminase, blood urea nitrogen, potassium, D-dimer and IL-6 were significantly higher in the mortality groups (P<0.05). However, according to the multivariate logistic regression, all these factors had similar values, proven by insignificant ORs in Table II.
| Parameters | Study participants (n=55) |
Severe (n=35) (Severe: n=17; Critical: n=18) |
Non-severe (n=20) (Mild: n=2; Moderate: n=18) |
p-value |
Non-survivor (n=22) (Non-severe: n=3; Severe: n=19) |
Survivor (n=33) (Non-severe: n=17; Severe: n=16) |
P-value |
|---|---|---|---|---|---|---|---|
| Age (yr) |
50.9±11.6 52 (45, 59) |
51.2±10.4 52.0 (46.0, 59.0) |
50.3±13.7 52.0 (43.5, 58.8) |
0.784a |
52.4±11.4 52.5 (47.8, 59.0) |
49.9±11.8 52.0 (43.5, 58.0) |
0.440a |
| Male, n(%) | 27 (49.1) | 16 (45.7) | 11 (55.0) | 0.508b | 11 (50.0) | 16 (48.5) | 0.912b |
| KL-6 (U/ml) |
48.0±26.1 40 (32.4, 56.7) |
42.7±20.4 37.7 (32.4, 48.2) |
57.3±32.4 44.1 (32.4, 82.5) |
0.130c |
43.7±22.1 40.4 (32.2, 50.5) |
50.9±28.4 39.1 (32.1, 71.7) |
0.757c |
| Blood gas analysis | |||||||
| pH |
7.4±0.1 7.4 (7.4, 7.4) |
7.4±0.1 7.4 (7.4, 7.4) |
7.4±0.1 7.4 (7.4, 7.5) |
0.958c |
7.4±0.1 7.4 (7.3, 7.4) |
7.4±0.1 7.4 (7.4, 7.5) |
0.003c |
| pO2 (mmHg) |
91.4±29.9 86.6 (66.8, 117.1) |
86.8±27.6 83.8 (62.1, 101.3) |
99.6±32.8 95.2 (73.6, 133.4) |
0.129a |
96.2±30.9 99.9 (68.4, 122.5) |
88.2±29.3 83.0 (65.3, 100.8) |
0.337a |
| pCO2 (mmHg) |
32.8±12.2 32.2 (25.9, 37) |
34.5±14.6 32.6 (25.9, 40.9) |
29.9±5.5 32.2 (26.0, 33.6) |
0.248c |
35.4±16.6 33.2 (24.9, 43.3) |
31.1±8.0 32.1 (26.2, 34.5) |
0.390c |
| BE (mEq/L) |
–3.7±4 –2.9 (–5.6, –1.2) |
–3.2±4.2 –2.5 (–5.2, –0.4) |
–4.7±3.5 –4.2 (–6.2, –2.4) |
0.192a |
–4.3±4.8 –3.3 (–7.3, –1.0) |
–3.4±3.4 –2.7 (–4.8, –1.3) |
0.378a |
| HCO3– (mEq/L) |
19.9±4.8 20.3 (17.9, 22.8) |
20.6±21.4 21.4 (18.3, 24.6) |
18.7±3.5 19.2 (17.8, 21.6) |
0.161a |
19.9±5.8 20.8 (15.7, 23.9) |
19.9±4.1 20.3 (18.3, 22.2) |
0.965a |
| AaDO2 (mmHg) |
325.2±201.5 369.5 (130.1, 485.3) |
403.3±158.5 439.1 (269.5, 539.4) |
188.6±198.9 82.2 (17.4, 401.9) |
<0.001a |
424.0±148.5 477.9 (300.2, 543.1) |
259.4±207.1 259.6 (54.5, 421.8) |
0.003c |
| SaO2 (%) |
94.9±5 96.5 (92.7, 98.5) |
94.4±5.0 96.3 (91.7, 97.9) |
95.8±5.0 97.4 (95.2, 99.0) |
0.115c |
94.5±6.0 97.3 (90.4, 98.8) |
95.2±4.4 96.3 (94.1, 97.7) |
0.777c |
| FiO2 (%) |
58.5±21 61.0 (33.0, 81) |
65.4±20.0 81.0 (50.0, 81.0) |
46.5±17.0 45.0 (33.0, 53.0) |
0.002c |
69.1±18.1 81.0 (59.0, 81.0) |
51.5±19.9 53.0 (33.0, 69.0) |
0.001c |
| P/F Ratio (mmHg) |
182.4±109 163.7 (106.9, 223.9) |
142.9±54.2 129.1 (102.5, 171.9) |
251.5±143.6 234.9 (153.8, 325.3) |
0.001c |
150.3±71.7 144.9 (102.2, 170.3) |
203.8±124.5 175.7 (108.6, 256.0) |
0.080c |
| Brixia score |
12.3±4.8 13.0 (8.0, 17.0) |
14.1±4.2 15.0 (12.0, 18.0) |
9.1±4.3 10. 0 (6.5, 12.0) |
<0.001c |
14.0±4.3 15.5 (11.0, 18.0) |
11.2±4.9 12.0 (7.5, 15.0) |
0.032c |
| Normal CXR, n(%) | 1 (1.8) | 0 (0.0) | 1 (5.0) | <0.001c | 0 (0.0) | 1 (3.0) | 0.026c |
| Mild changes, n(%) | 6 (10.9) | 2 (5.7) | 4 (20) | 2 (9.1) | 4 (12.1) | ||
| Moderate changes, n(%) | 19 (34.5) | 7 (20.0) | 12 (60.0) | 4 (18.2) | 15 (45.5) | ||
| Severe changes, n(%) | 29 (52.7) | 26 (74.3) | 3 (15) | 16 (72.7) | 13 (39.4) |
Continuous variables are shown as mean±SD and median [interquartile range (IQR)]. The comparison between two groups, either severe vs. non-severe and non-survivor vs. survivor, was performed using an independent at-test, bChi-square test, cMann–Whitney U test, or Fisher’s exact test. AaDO2, alveolar-to-arterial oxygen pressure gradient; BE, base excess; CXR, chest X-ray; FiO2, fraction of inspired oxygen; HCO3–, bicarbonate ion concentration; KL-6, Krebs von den Lungen-6; P/F ratio, PaO2/FiO2 ratio; pCO2, partial carbon dioxide pressure; pO2, partial oxygen pressure; SaO2, arterial oxygen saturation; AaDO2, alveolar-to-arterial oxygen pressure gradient.
| Parameters | Severe vs. Non-severe | Non-survivor vs. Survivor | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | |
|
Respiratory rate (breaths/min) |
<0.001 | 1.420 (1.179–1.71) | 0.007 | 1.567 (1.130–2.174) | 0.011 | 1.171 (1.037–1.323) | 0.355 | 1.094 (0.904–1.325) |
|
Red blood cell (106/ml) |
0.036 | 2.532 (1.062–6.039) | 0.077 | 3.614 (0.869–15.037) | ||||
|
Platelet (103/ml) |
0.034 | 1.007 (1.001–1.013) | 0.105 | 1.011 (0.998–1.025) | ||||
| SGOT(U/L) | 0.107 | 1.010 (0.998–1.022) | ||||||
| BUN (mg/dl) | 0.025 | 1.087 (1.010–1.169) | 0.208 | 1.080 (0.958–1.217) | ||||
| Potassium (mmol/L) | 0.004 | 6.672 (1.847–24.101) | 0.236 | 3.291 (0.459–23.613) | ||||
| D-Dimer (mcg/ml) | 0.07 | 2.831 (0.918–8.732) | 0.259 | 1.074 (0.949–1.215) | ||||
| Interleukin-6 (pg/ml) (N/A=13) | 0.060 | 1.006 (1–1.012) | ||||||
| C-reactive protein (mg/L) | 0.011 | 1.010 (1.002–1.018) | 0.114 | 1.011 (0.997–1.024) | ||||
| pH | 0.113 | 0.008 (0.000–3.159) | ||||||
| AaDO2 (mmHg) | 0.001 | 1.006 (1.003–1.010) | 0.362 | 1.003 (0.997–1.010) | 0.005 | 1.005 (1.001–1.008) | 0.422 | 1.002 (0.997–1.008) |
| FiO2(%) | 0.002 | 1.050 (1.018–1.083) | 0.736 | 1.014 (0.937–1.096) | 0.003 | 1.048 (1.015–1.081) | 0.091 | 1.043 (0.993–1.095) |
| P/F ratio (mmHg) | 0.002 | 0.986 (0.978–0.995) | 0.720 | 0.997 (0.981–1.013) | ||||
| Brixia score | 0.001 | 1.294 (1.111–1.507) | 0.031 | 1.401 (1.032–1.903) | 0.037 | 1.149 (1.008–1.310) | 0.473 | 1.093 (0.857–1.393) |
BUN, blood urea nitrogen; CI, confidence interval; OR, odds ratio
Diagnostic value of KL-6, BGA and Brixia score in COVID-19 severity and mortality
Table I provided the KL-6, BGA parameters and Brixia score. No significant differences in the severity and mortality groups were observed in KL-6, pCO2, BE, HCO3– and SaO2. However, alveolar-arterial oxygen gradient, FiO2 and Brixia score were significantly different in the severity and mortality groups. We also performed multivariate logistic regression (Table II). The result showed that only Brixia score was significantly higher in severe participants as compared to the non-severe ones (OR 1.401 [1.032–1.903]; P=0.031). There was no significant correlation between KL-6 and several BGA parameters in COVID-19 severity (Fig. 1A-E) and mortality (Fig. 2A-E). Nevertheless, the strongest KL-6-BGA correlations were observed between KL-6 and HCO3– in the severe group (r=0.370, Fig. 1D) and between KL-6 and pCO2 in the survivor group (r=0.224, Fig. 2B). Interestingly, KL-6 and Brixia scores had a significantly negative correlation overall among all COVID-19 positive individuals (r=–0.283; P=0.036), as shown by the black line in Figs. 1F and 2F. In separated analysis, KL-6 and Brixia score were significantly correlated in the COVID-19 survivors (r=–0.347; P=0.048, Fig. 2F), but not in the non-severe group (r= –0.285; P=0.223, Fig. 1F).

- Correlation strengths between KL-6 and several COVID-19 severity parameters of BGA and CXR. (A) pH, (B) pCO2, (C) BE, (D) HCO3–, (E) P/F ratio and (F) Brixia score. BE, base excess; BGA, blood gas analysis; COVID-19, Coronavirus disease 2019; CXR, chest X-ray; KL-6, Krebs von den Lungen-6; P/F ratio, PaO2/FiO2 ratio; pCO2, partial carbon dioxide pressure.
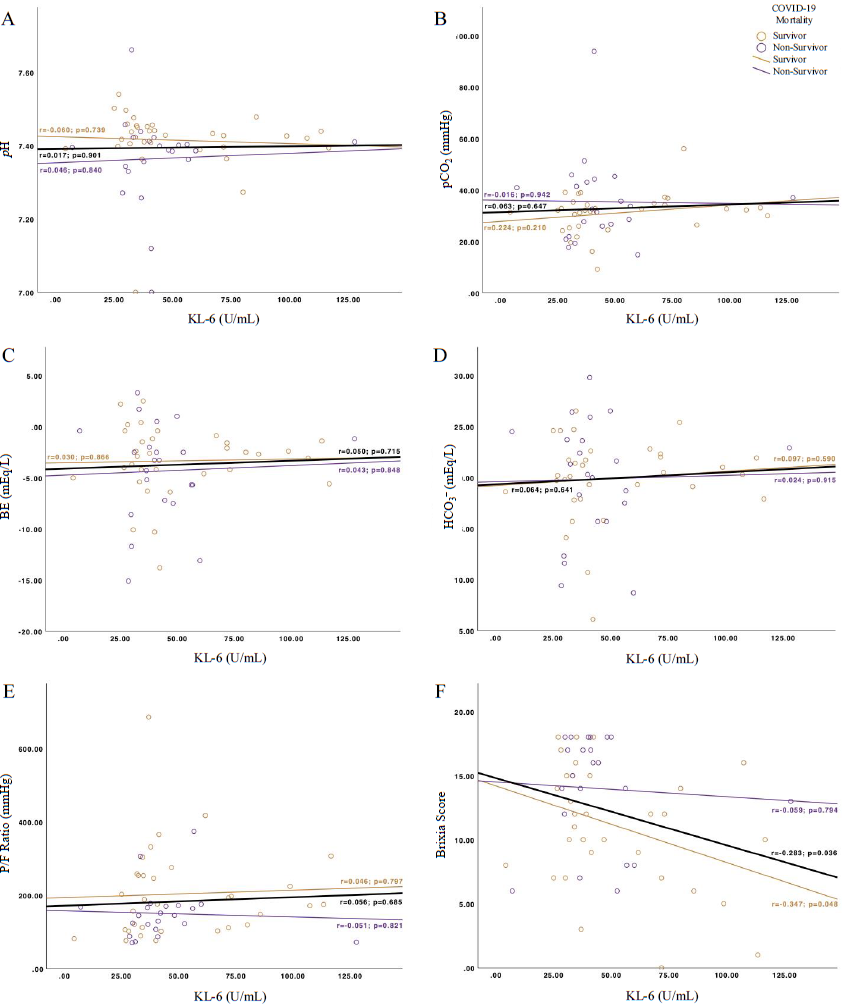
- Correlation strengths between KL-6 and several COVID-19 mortality parameters of BGA and CXR. (A) pH, (B) pCO2, (C) BE, (D) HCO3–, (E) P/F ratio and (F) Brixia score.
ROC analyses to assess the diagnostic value of KL-6, P/F ratio and Brixia score in COVID-19 severity and mortality are shown in Figure 3 and Table III. In this study, the AUCs of P/F ratio were 0.764 (0.614–0.915) in the severe group and 0.64 (0.491–0.79) in non-survivors. Even though KL-6 had a low AUC in determining COVID-19 severity (0.376 [0.212–0.541]) and mortality (0.475 [0.320–0.630]), KL-6 might still be beneficial to improve the AUC of P/F ratio. The AUC increase was found when adding KL-6 to the P/F ratio analysis, either in severe participants (0.764–0.8) or in non-survivors (0.640–0.652). Interestingly, adding KL-6 to P/F ratio showed an increase in specificity in the ‘severe’ group from 0.35 to 0.75 while also increasing its sensitivity (0.886–0.971), but not in non-survivors. Furthermore, the Brixia score had the highest AUC in the severe group [0.801 (0.685–0.917)] and non-survivors [0.671 (0.522–0.82)], with both having a similar optimal cut-off (≥12.5). We analysed the AUC of the Brixia score using our pre-defined cut-off (≥7) according to the previously stated classification in the methods section and Table III. The AUC increase of Brixia score was only observed in the severity analysis, but not in the mortality. Of those analyses, KL-6 and Brixia score together served as the best severity markers in this study (AUC 0.809 [0.697–0.920]; Sn/Sp=0.686/0.900), followed by KL-6 and P/F ratio (AUC 0.800 [0.637–0.963]; Sn/Sp=0.971/0.75).
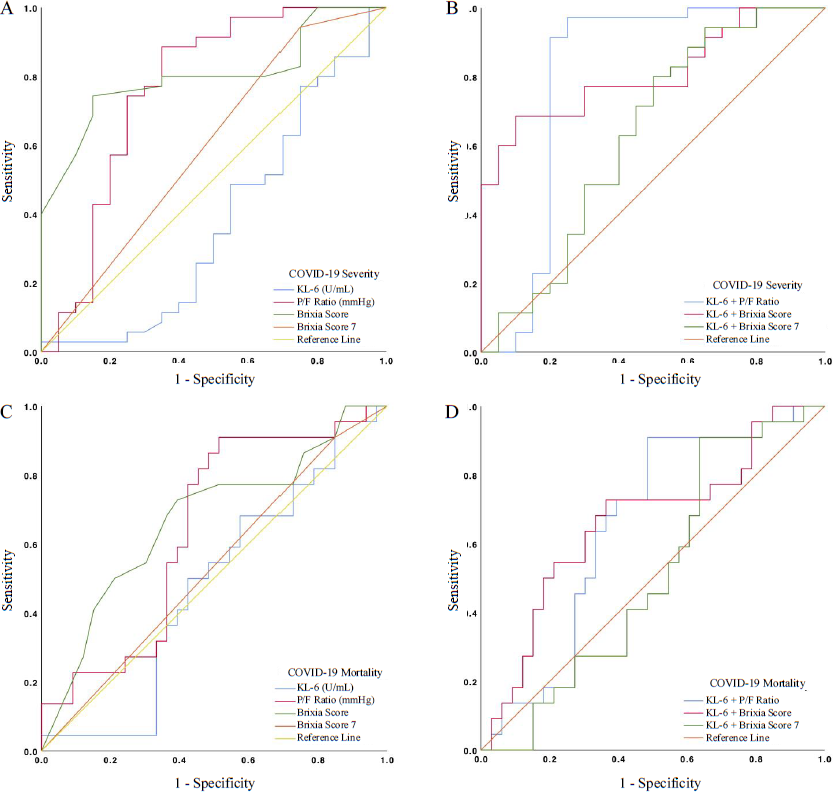
- ROC curve of KL-6, P/F ratio, Brixia score and their combinations in COVID-19 severity (A, B) and mortality (C, D). ROC, receiver operating characteristic.
| Parameters | AUC (95% CI) | P-value | Optimal cut-off | Sensitivity | Specificity | Youden’s Index |
|---|---|---|---|---|---|---|
| COVID-19 severity (severe) | ||||||
| KL-6 | 0.376 (0.212–0.541) | 0.13 | ≥5.732 | 1.000 | 0.050 | 0.050 |
| P/F ratio | 0.764 (0.614–0.915) | 0.001 | ≤190.515 | 0.886 | 0.350 | 0.536 |
| Brixia score | 0.801 (0.685–0.917) | <0.001 | ≥12.500 | 0.743 | 0.850 | 0.593 |
| Brixia score ≥7 | 0.596 (0.434–0.758) | 0.238 | N/A | 0.943 | 0.250 | 0.193 |
| KL-6+P/F ratio | 0.800 (0.637–0.963) | <0.001 | N/A | 0.971 | 0.750 | 0.721 |
| KL-6+Brixia score | 0.809 (0.697–0.920) | <0.001 | N/A | 0.686 | 0.900 | 0.586 |
| KL-6+Brixia score ≥7 | 0.630 (0.463–0.797) | 0.111 | N/A | 0.800 | 0.500 | 0.300 |
| COVID-19 mortality (non-survivors) | ||||||
| KL-6 | 0.475 (0.320–0.630) | 0.757 | ≥28.388 | 0.955 | 0.152 | 0.106 |
| ≥35.681 | 0.682 | 0.424 | 0.106 | |||
| P/F ratio | 0.640 (0.491–0.79) | 0.080 | ≤182.850 | 0.909 | 0.515 | 0.394 |
| Brixia score | 0.671 (0.522–0.82) | 0.033 | ≥12.500 | 0.727 | 0.606 | 0.333 |
| Brixia score ≥7 | 0.530 (0.375–0.686) | 0.705 | N/A | 0.909 | 0.152 | 0.061 |
| KL-6+P/F ratio | 0.652 (0.504–0.799) | 0.059 | N/A | 0.909 | 0.515 | 0.424 |
| KL-6+Brixia score | 0.663 (0.512–0.813) | 0.043 | N/A | 0.727 | 0.636 | 0.496 |
| KL-6+Brixia score ≥7 | 0.51 (0.356–0.663) | 0.904 | N/A | 0.909 | 0.364 | 0.273 |
AUC, area under curve
Discussion
So far, the authors believe that this is the first study from Indonesia to report the significance of KL-6 and its correlation with BGA and Brixia score and their ROC analyses in COVID-19 severity and mortality. Although no significant difference in KL-6 was found in severe vs. non-severe participants or non-survivors vs. survivors. However, in the multivariate analysis, we found that RR and Brixia scores were significantly higher in the severe patients compared to the non-severe patients. In the KL-6-BGA analysis, a positive correlation was found between KL-6 and HCO3– in the severe group and KL-6 and pCO2 in the survivor group. KL-6 expression and Brixia scores were inversely correlated in all the samples. Despite its non-significant difference and its low AUC in both severity and mortality, KL-6 was found to increase the P/F ratio diagnostic value in determining COVID-19 severity or mortality. KL-6 and Brixia score only had a noticeable increase of diagnostic value in COVID-19 severity, but not in mortality. These findings suggested that KL-6 might still be used in reflecting the extent of COVID-19 severity and mortality.
This study found that KL-6 expression were not significantly different, between the COVID-19 severity and mortality groups. Moreover, in this study KL-6 expression was lower than in the previous studies17,18. Several reasons might explain both results. The probable explanation was the different reagents used. We conducted the serum KL-6 investigation using a similar ELISA reagent kit as used by Suryananda and Yudhawati5. KL-6 levels were similar, ∼40–50 U/ml. Two studies by Awano et al17 and Xue et al18 were observed using two different reagents, resulting in a higher serum KL-6, ∼200–600 U/ml.
Several previous studies19,20 found that KL-6 might also be related to the individual’s ethnicity and genotype. Horimasu et al19 found that KL-6 was higher in the German population than in the Japanese. Furthermore, cases of individuals with ILD serum KL-6 was higher among those with A/G and G/G genotypes compared to the A/A genotype. The allele variation also influenced this difference in some individuals19. Those with the MUC1 rs4072037 CC genotype might have higher KL-6 due to the disequilibrium abundance of tandem repeats in MUC119,20. Thus, we might assume that the survivors and non-severe participants probably have this single nucleotide polymorphism, which leads to a higher serum KL-6, even in healthy conditions. Furthermore, Suryananda and Yudhawati5 stated similar results as ours, indicating that Indonesian people certainly had a low serum KL-6.
Strict COVID-19 management might reduce lung injury sequelae and be related to low KL-6. It might also be used as a successful indicator of aggressive interventions21. Gender differences might also affect KL-6 results. Even though sex was not significantly different in our study, 55 per cent of males had non-severe COVID-19, resulting in a higher level of KL-6 expression. This was similar to the study by Suryananda and Yudhawati5, with 61.3 per cent of the participants being males. As mentioned earlier, KL-6 levels might change dynamically due to the affected lung area combined with the possibility of a genetic polymorphism18,20. In this study, the onset of the disease, the time of blood samples taken, the severity during admission and the disease progression rates might have lead to an earlier treatment; due to which serum KL-6 levels were lower than in other studies.
This study showed that KL-6 was not significantly correlated with the BGA. This might be caused by the aggressive COVID-19 treatment, which affected KL-6 and BGA21. Another study suggested that KL-6 levels tended to remain unchanged during mild lung injury compared to other biomarkers, indicating that KL-6 only increased when there was severe alveolar damage and an increase in alveolar permeability; thus, might create a discrepancy between KL-6 and BGA22. Nevertheless, this study demonstrated a strong correlation between KL-6 and HCO3–. The HCO3– levels remained unchanged in severe conditions for 90 days. Interestingly, persistent KL-6 levels in the severe participants could be observed until 56 days. This might explain our finding related to KL-6 and HCO3–18,23. We also found a significant negative correlation between KL-6 and Brixia score. However, to date, no studies have discussed KL-6 and Brixia scores. This finding might be affected by the contradictory low serum KL-6 and high Brixia score in our study population.
Our results contradicted the previous studies conducted in East Asia and Europe, where KL-6 was shown to have a high diagnostic value in evaluating COVID-19 severity (AUC 0.824–0.850) and mortality (AUC 0.849)1,4,17,24,25. Their optimal cut-off values varied from 278.3 to 406.5 U/ml, while the cut-offs in this study ranged from 5.7 to 35.7 U/ml. These values were expected due to our study’s lower mean of KL-6 as compared to other studies. Again, we speculated that the differences in reagent kits, population characteristics and racial ethnicities might cause the discrepancy in the KL-6 diagnostic performance for COVID-19 severity and mortality. Thus, more extensive studies involving a specific type of population are needed to test this hypothesis and confirm our findings.
Unlike KL-6, our ROC analysis on the P/F ratio and Brixia score aligned with earlier research. Gu et al26 found that the P/F ratio had a high performance in predicting COVID-19 mortality in the intensive care ward, while Sinatti et al27 revealed that the P/F ratio was reliable for identifying severe COVID-19 individuals with oxygen support. For predicting COVID-19 in-hospital mortality, Gatti et al28 found that the Brixia score had an AUC of 0.81. However, combining KL-6 did not seem to benefit its diagnostic value, neither using our optimal cut-off nor the pre-defined cut-off. This was proven by our significant multivariate analysis, which showed that the Brixia score is an independent factor in determining COVID-19 severity. The most apparent AUC increase of the P/F ratio was observed when incorporating KL-6 into the severity analysis. Nevertheless, we encourage more research evaluating the KL-6 combination with P/F ratio and Brixia score, especially in East Asia and Europe, given that KL-6 in these populations was shown to have a high diagnostic value for COVID-19.
This study had several limitations. First, a single-centred cross-sectional study with limited participants, might affect the statistical analysis. Second, some missing values <5 per cent on several variables were replaced using multiple imputations. This still could not eliminate or even reduce the issue around the small sample size. We also did not achieve this method for variables with missing values ≥5 per cent to avoid further bias in the analysis. Third, there was a difference in the time interval between the hospital admission, the blood sampling and the time of CXR taken for each participant. At this interval, the participants received different therapeutic approaches with extra length of time to maintain their best clinical conditions. This might have affected the KL-6 levels, BGA results and Brixia scores.
In conclusion, KL-6 has the potential to be an adjunct laboratory parameter to BGA and Brixia scores representing COVID-19 severity and mortality. KL-6 and P/F ratio could be used as promising diagnostic tools since their combination yielded high sensitivity and specificity, either in COVID-19 severity or mortality analyses. KL-6 also had the strongest correlation with HCO3–, which might both be maintained at a certain level to depict COVID-19 lung injury. Furthermore, even though combining KL-6 did not seem to benefit the diagnostic value of the Brixia score, it still served as the best COVID-19 severity marker in this study. Future studies involving a larger population are needed to confirm the relationship between KL-6, BGA and Brixia scores. We also suggest that more future research is needed to analyze the comparison between different reagent kits of KL-6 since they may affect the values of KL-6.
References
- Serum Krebs von den Lungen-6 for predicting the severity of COVID-19 lung injury: A systematic review and meta-analysis. Iran Biomed J. 2021;25:381-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Lung fibrosis sequelae after recovery from COVID-19 infection. J Infect Dev Ctries. 2021;1:360-5.
- [CrossRef] [Google Scholar]
- Detection of COVID-19 severity using blood gas analysis parameters and Harris hawks optimized extreme learning machine. Comput Biol Med. 2022;142:105166.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum KL-6 could represent a reliable indicator of unfavourable outcome in patients with COVID-19 pneumonia. Int J Env Res Public. 2021;18:1-10.
- [CrossRef] [Google Scholar]
- Association of serum KL-6 levels on COVID-19 severity: A cross-sectional study design with purposive sampling. Ann Med Surg. 2021;69:102673.
- [CrossRef] [Google Scholar]
- Krebs Von den Lungen-6 as a predictive indicator for the risk of secondary pulmonary fibrosis and its reversibility in COVID-19 patients. Int J Biol Sci. 2021;17:1565-73.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Correlating arterial blood gas, acid-base and blood pressure abnormalities with outcomes in COVID-19 intensive care patients. Ann Clin Biochem. 2021;58:95-101.
- [CrossRef] [PubMed] [Google Scholar]
- COVID-19: Current understanding of its pathophysiology, clinical presentation and treatment. Postgr Med J. 2021;97:312-20.
- [CrossRef] [Google Scholar]
- COVID-19 outbreak in Italy: Experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020;125:509-13.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Radiographic severity index in COVID - 19 pneumonia : relationship to age and sex in 783 Italian patients. Radiol Med. 2020;125:461-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognostic value of Charlson Comorbidity Index in the elderly with a cardioverter defibrillator implantation. Int J Cardiol Netherlands. 2020;314:64-9.
- [CrossRef] [Google Scholar]
- BS-Net: learning COVID-19 pneumonia severity on a large chest X-ray dataset. Med Image Anal. 2021;71:102046.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The relationship of chest X-ray in COVID-19 patients and disease severity in Arifin Achmad General Hospital Riau. J Respir. 2021;7:114-21.
- [CrossRef] [Google Scholar]
- Burhan E, Susanto AD, Isbaniah F, Nasution SA, Ginanjar E, Pitoyo CW, eds. Pedoman Tatalaksana COVID-19 (3rd ed). Jakarta: PDPI, PERKI, PAPDI, PERDATIN, IDAI; 2020. p. :1-138.
- Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. 2011;20:40-9.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The proportion of missing data should not be used to guide decisions on multiple imputation. J Clin Epidemiol. 2019;110:63-73.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum KL-6 level is a useful biomarker for evaluating the severity of coronavirus disease 2019. Respir Investig. 2020;58:440-7.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Exploration and correlation analysis of changes in Krebs von den Lungen-6 levels in COVID-19 patients with different types in China. Biosci Trends. 2020;14:290-6.
- [CrossRef] [PubMed] [Google Scholar]
- Different MUC1 gene polymorphisms in German and Japanese ethnicities affect serum KL-6 levels. Respir Med. 2012;106:1756-64.
- [CrossRef] [PubMed] [Google Scholar]
- Role of MUC1 rs4072037 polymorphism and serum KL-6 levels in patients with antisynthetase syndrome. Nature. 2021;11:22574.
- [Google Scholar]
- Review and analysis of current responses to COVID-19 in Indonesia: Period of January to March 2020. Prog Disaster Sci. 2020;6:100091.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Serum krebs von den lungen-6 (KL-6): a promising biomarker in sarcoidosis. MOJ Curr Res Rev. 2018;1:44-7.
- [CrossRef] [Google Scholar]
- Bicarbonate concentration as a predictor of prognosis in moderately severe COVID-19 patients: A multicenter retrospective study. PLoS One. 2022;17:e0270141.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Correlation of Krebs von den Lungen-6 and fibronectin with pulmonary fibrosis in coronavirus disease 2019. Clin Chim Acta. 2021;517:48-53.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Prognostic bioindicators in severe COVID-19 patients. Cytokine. 2021;141:155455.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- PaO2/FiO2 and IL-6 are risk factors of mortality for intensive care COVID - 19 patients. Sci Rep. 2021;11:7334.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- PaO2/FiO2 ratio forecasts COVID-19 patients’ outcome regardless of age: a cross-sectional, monocentric study. Intern Emerg Med. 2022;17:665-73.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Emergency room comprehensive assessment of demographic, radiological laboratory and clinical data of patients with COVID-19: Determination of its prognostic value for in-hospital mortality. Intern Emerg Med. 2022;17:205-14.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]






